Synthetic method of hindered phenol/hindered amine intramolecular compound anti-oxidant
A synthesis method and hindered amine technology, which are applied in the fields of fine chemicals and polymer materials, can solve the problems of few reports on the compounding of hindered phenols and hindered amine light stabilizers, and achieve a product with excellent performance, high yield, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
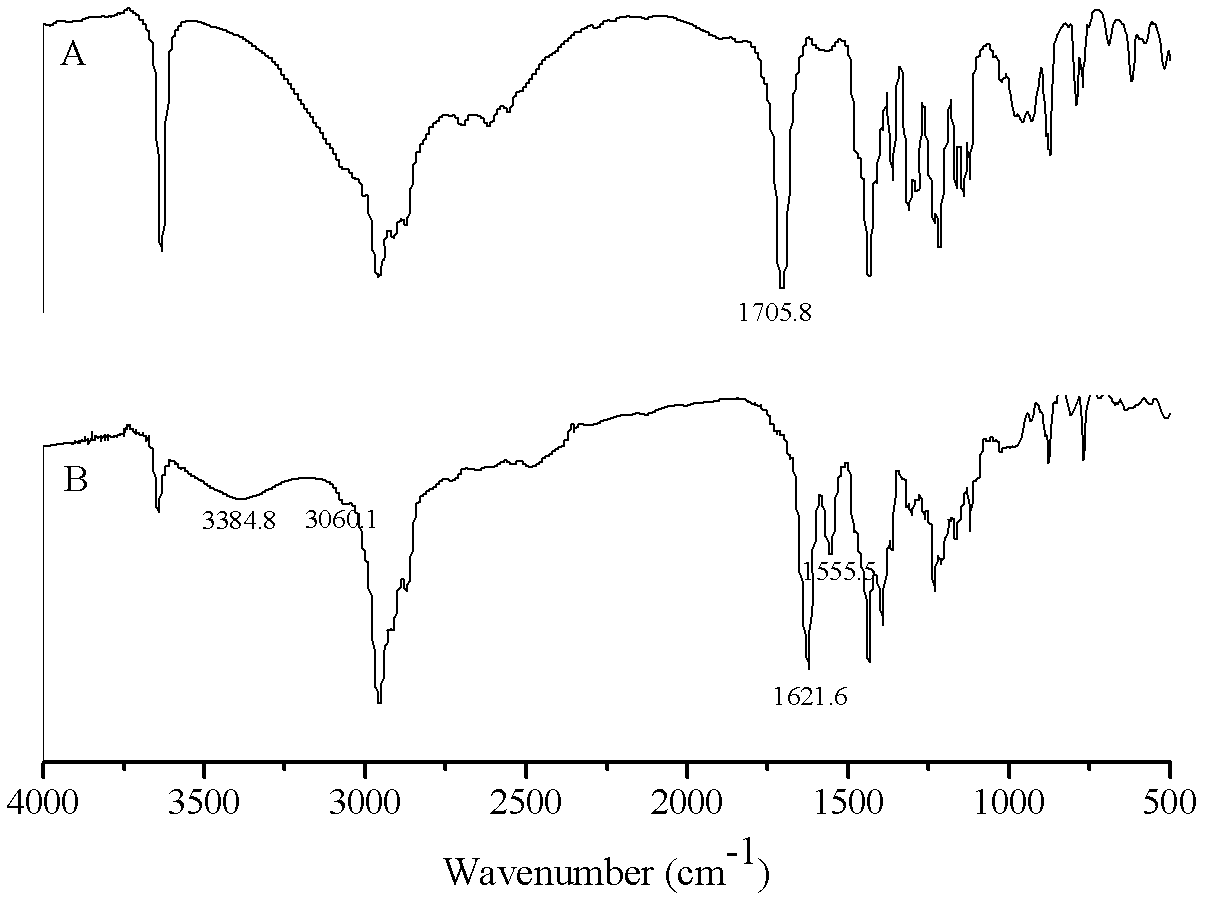
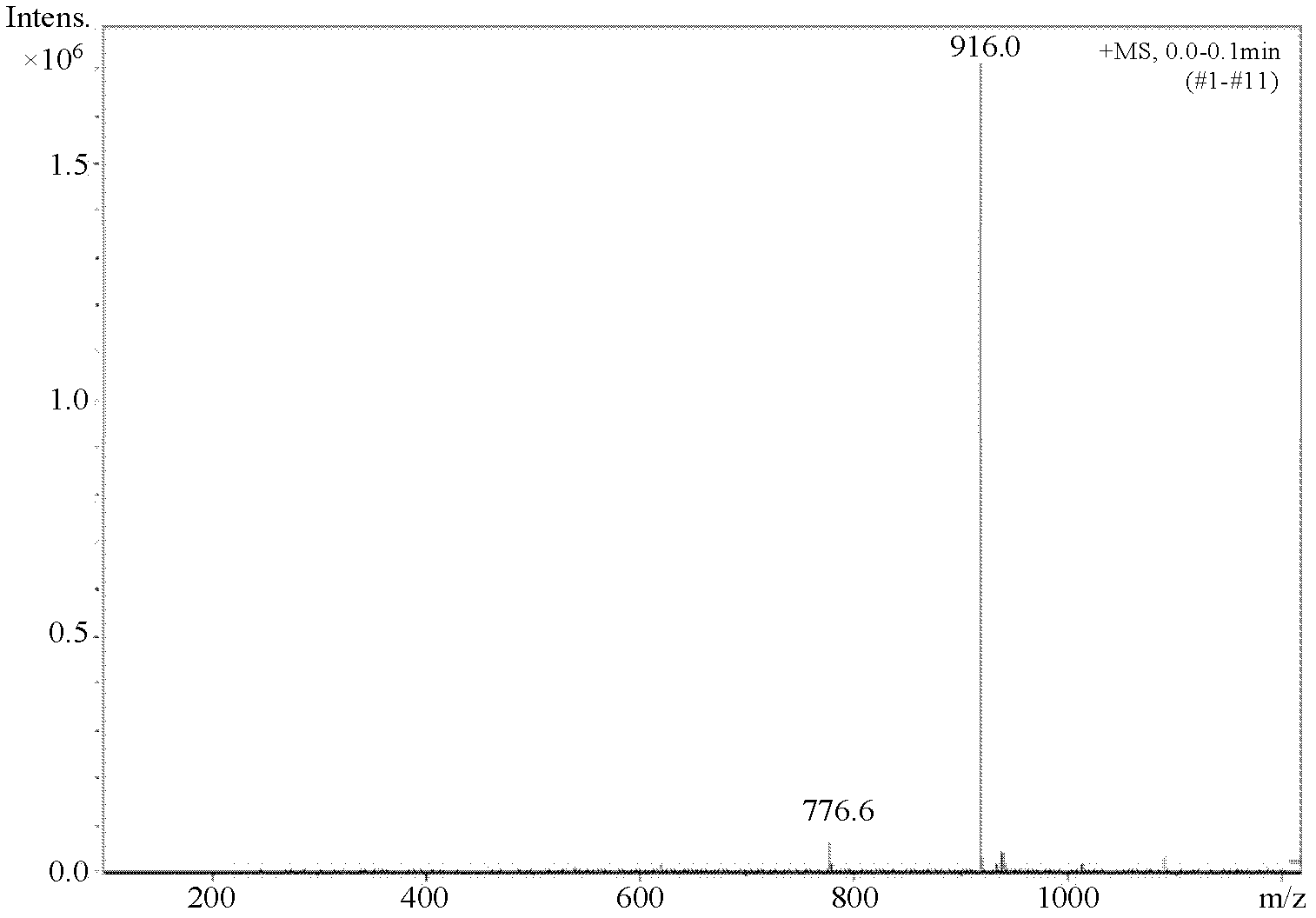
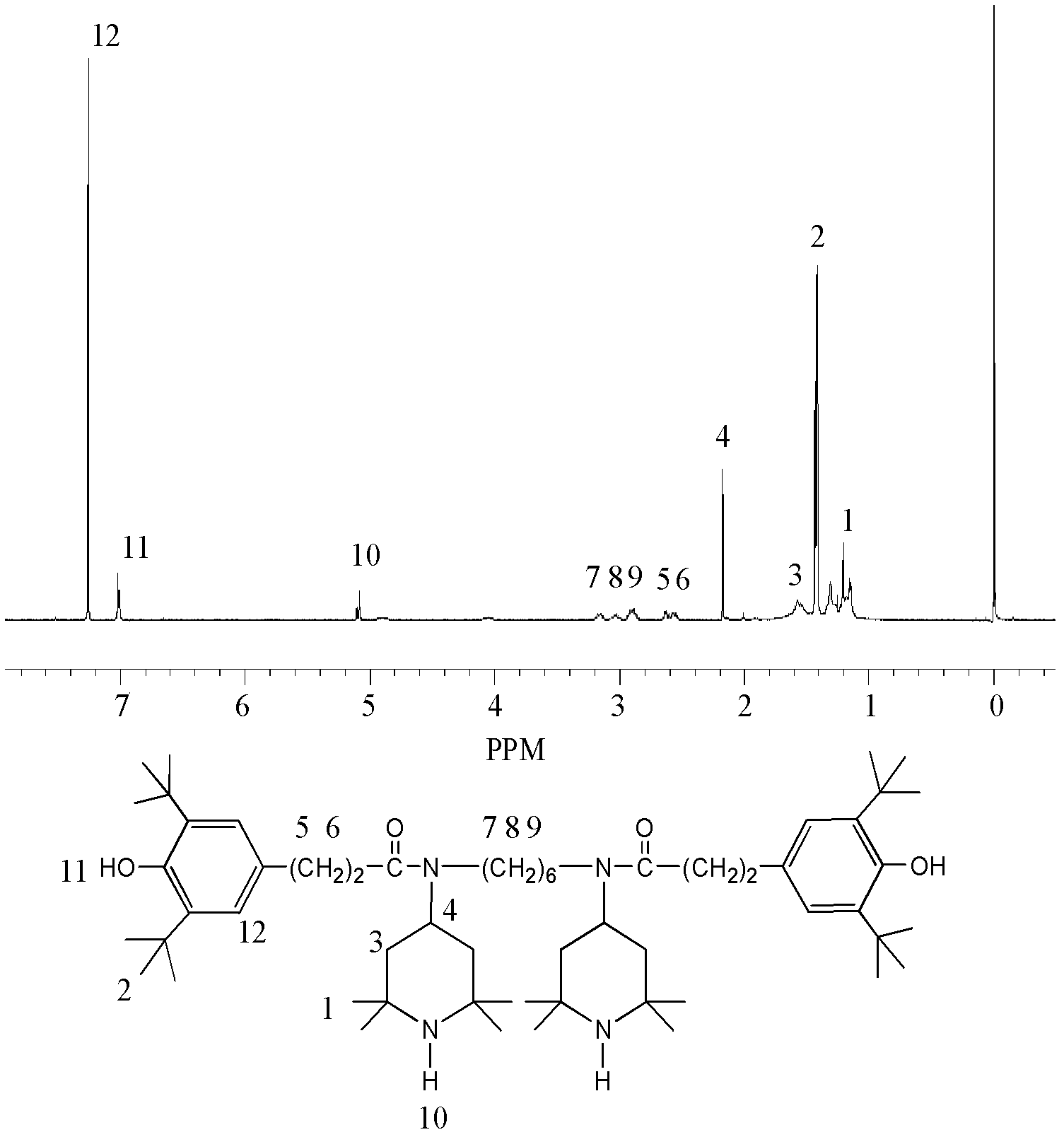
[0044] Accurately weigh 5.56g (0.02mol) of β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid, put it into a three-necked bottle, add 50mL of chloroform to dissolve, and then place it at a constant temperature of 50°C In a magnetically stirred water bath, slowly add 3.6 mL (0.05 mol) of thionyl chloride dropwise with a constant pressure dropping funnel to carry out a substitution reaction, stop the reaction after 5 hours, and distill under reduced pressure to remove chloroform and unreacted thionyl chloride. Pale yellow crystals of β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl chloride were obtained with a yield of 98.2%.
[0045] Dissolve 5.94g (0.02mol) of β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl chloride in 50mL of acetone and put it into a three-neck flask, cool to 0-5°C in an ice bath, and stir Slowly add 3.95g (0.01mol) of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-hexanediamine in acetone solution, after the addition After reacting for 1 h, 4 mL of aqueous...
Embodiment 2
[0051] Accurately weigh 5.56g (0.02mol) of β-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid, put it into a three-necked bottle, add 50mL of dichloromethane to dissolve, and then place it at a constant temperature of 50°C In a magnetic stirring water bath, slowly add 2.88mL (0.04mol) thionyl chloride dropwise with a constant pressure dropping funnel to carry out the substitution reaction, stop the reaction after 4.5h, and distill under reduced pressure to remove dichloromethane and unreacted thionyl chloride , to obtain β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl chloride as pale yellow crystals with a yield of 98.6%.
[0052] Dissolve 5.94g (0.02mol) of β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl chloride in 50mL of toluene and put it into a three-neck flask, cool it down to 0-5°C in an ice bath, and stir Slowly add 3.95g (0.01mol) of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-hexanediamine in toluene solution, after the addition React for 2 hours; then slowly ...
Embodiment 3
[0054] Accurately weigh 5.28g (0.02mol) β-(3,5-di-tert-butyl-4-hydroxyphenyl) acetic acid, put it into a three-necked bottle, add 50mL N, N'-dimethylformamide to dissolve, and then Place in a constant temperature magnetically stirred water bath at 60°C, slowly add 2.16mL (0.03mol) thionyl chloride dropwise with a constant pressure dropping funnel to carry out the substitution reaction, stop the reaction after 4h, and distill under reduced pressure to remove N,N'-dichloride Methylformamide and unreacted thionyl chloride gave light yellow crystals of β-(3,5-di-tert-butyl-4-hydroxyphenyl)acetyl chloride with a yield of 96.7%.
[0055] Dissolve 5.64g (0.02mol) of β-(3,5-di-tert-butyl-4-hydroxyphenyl) acetyl chloride in 50mL of acetone and put it into a three-neck flask, cool to 0-5°C in an ice bath, and stir Slowly add 3.95g (0.01mol) of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-hexamethylenediamine in acetone solution, after the addition After reacting for 1 h, 4.5 mL of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com