Rhodamine derivatives and their preparation method and use
A preparation and compound technology, applied in the field of rhodamine derivatives, can solve the problems of being unsuitable for real-time quantitative research on living cells, short reaction stabilization time, and small concentration range, etc., achieving shortened reaction stabilization time, high sensitivity, and fast stabilization speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
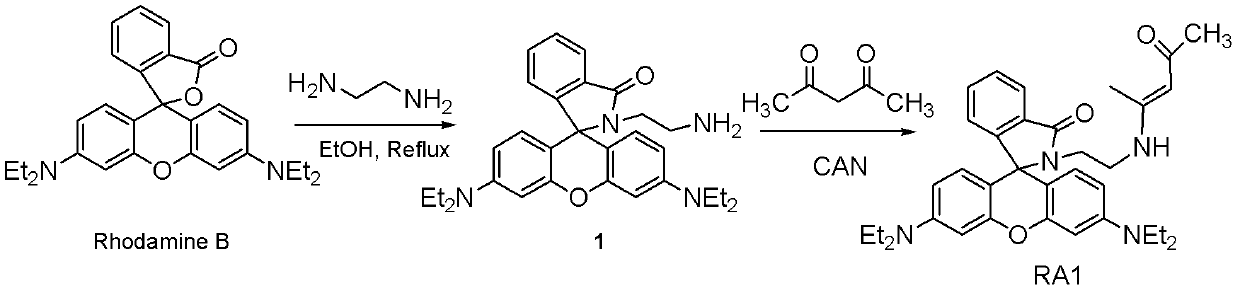
[0048] Preparation of Compound 1, namely (E)-3',6'-bis(diethylamino)-2-(2-(4-oxopent-2-en-2-ylamino)ethyl)spiro[isoind Indoline-1,9'-oxanthene]-3-one.
[0049] Rhodamine B (2.4g, 5mmol) was dissolved in 60mL of ethanol solution into a 250ml round bottom flask, and 0.43mL of ethylenediamine was added dropwise with stirring at room temperature. After the dropwise addition, heat (the boiling temperature of ethanol is 78.5° C.) to reflux for 12 hours, and the reaction system becomes clear until the detection by thin layer chromatography (TLC) shows that the raw materials disappear. After cooling to room temperature, the solvent-ethanol was removed under reduced pressure. Add about 1mol / L 100mL HCl solution to remove unreacted ethylenediamine, then slowly neutralize with NaOH to pH 9-10, extract with dichloromethane, anhydrous Na 2 SO 4 After drying, the resulting solid was washed with CHCl 3 / MeOH (98:2, V:V) column chromatography (the "column" used was a silica gel column) wi...
Embodiment 2
[0056] Embodiment 2, preparation compound RA2, method is the same as embodiment 1, just replaces acetylacetone with ethyl acetoacetate in the reaction raw material, and its nuclear magnetic resonance parameter is:
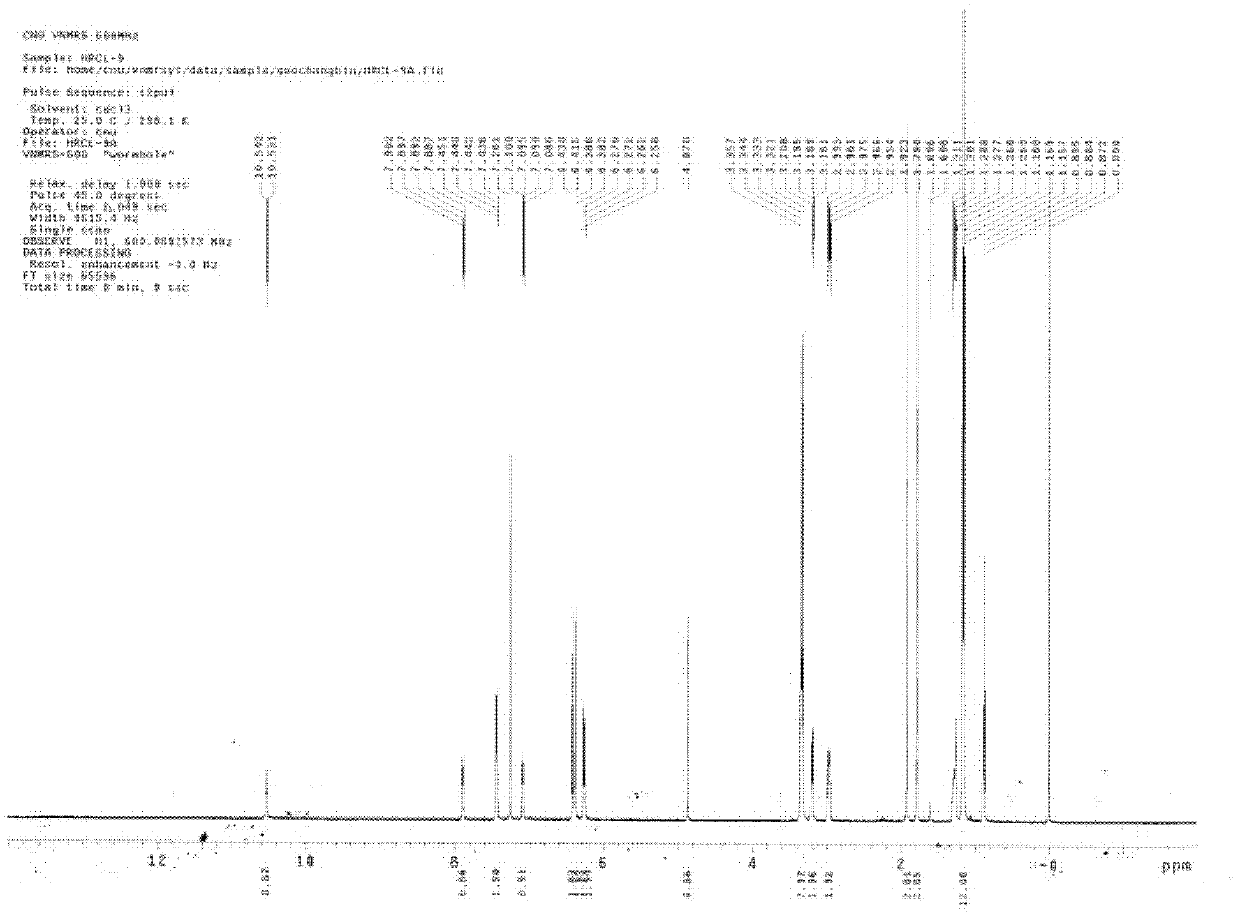
[0057] 1 H NMR (400MHz, CDCl 3 ): δ1.15-1.19 (m, 15H, CH 2 CH 3 ), 1.76 (s, 3H, CH 3 ), 2.95(t, 2H, J=4.4Hz, CH 2 CH 2 ), 3.18(t, 2H, J=4.4Hz, CH 2 CH 2 ), 3.30-3.36 (m, 4H, NCH 2 CH 3 ), 4.02(q, 2H, J=7.2Hz, OCH 2 CH 3 ), 4.34 (s, 1H, C=CH), 6.24-6.43 (m, 6H, Ar), 7.08-7.10 (m, 1H, Ar), 7.43-7.45 (m, 2H, Ar), 7.89-7.91 ( m, 1H, Ar), 8.38 (t, 1H, J=6.4Hz, NH);
[0058] 13 C NMR (100MHz, CDCl 3 ): 12.5, 18.2, 19.5, 28.6, 40.2, 41.6, 44.5, 64.6, 95.5, 97.6, 105.1, 108.2, 122.6, 123.7, 128.3, 131.1, 132.4, 148.9, 153.2, 153.5, 163.2, 167.6.8, 19
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com