Cyclometalated iridium complex organic electrophosphorescent material, its preparation method and application
A technology of iridium complexes and phosphorescent materials, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., to achieve the effect of simple synthesis method and high quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
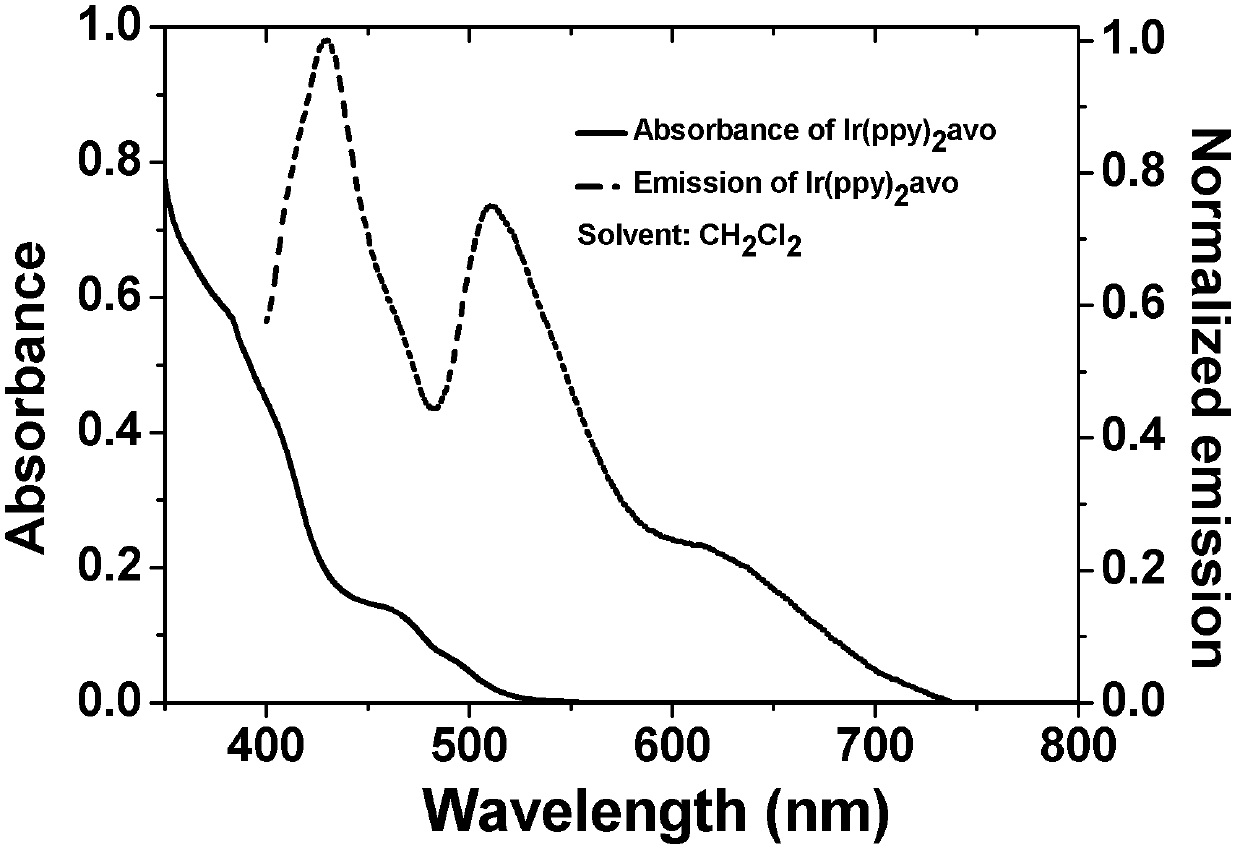
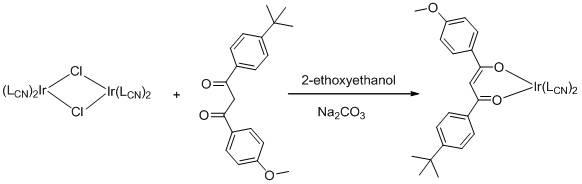
[0027] Example 1 Weigh 428 mg [Ir(ppy) 2 Cl] 2 , 310 mg of avobenzone and 211 mg of anhydrous sodium carbonate were added to 50 mL of ethylene glycol monoethyl ether, under nitrogen protection, refluxed for 24 hours, then allowed to stand at room temperature, the solvent was spin-dried, and dichloro Methane:petroleum ether=2:1, separated and purified by column chromatography to obtain 500 mg of the target product. Yield: 77%. The product is characterized by NMR, and the results are as follows: 1 H NMR in CDCl 3 , 8.59 (dd, J=6,10 Hz, 2 H), 7.79 (m, 6 H), 7.63 (m, 2 H), 7.57 (m, 2 H), 7.31 (d, J=7.6 Hz, 2 H), 7.00(m, 2H), 6.84(m, 2H), 6.78(m, 2H), 6.72(m, 2H), 6.53(s, 1H), 6.36(t, J=7.6 Hz, 2H), 3.78(s, 3H), 1.27(s, 9H). MS (EI): m / z 810 (M + ). The spectral properties of the product are as follows figure 1 shown.
Embodiment 2
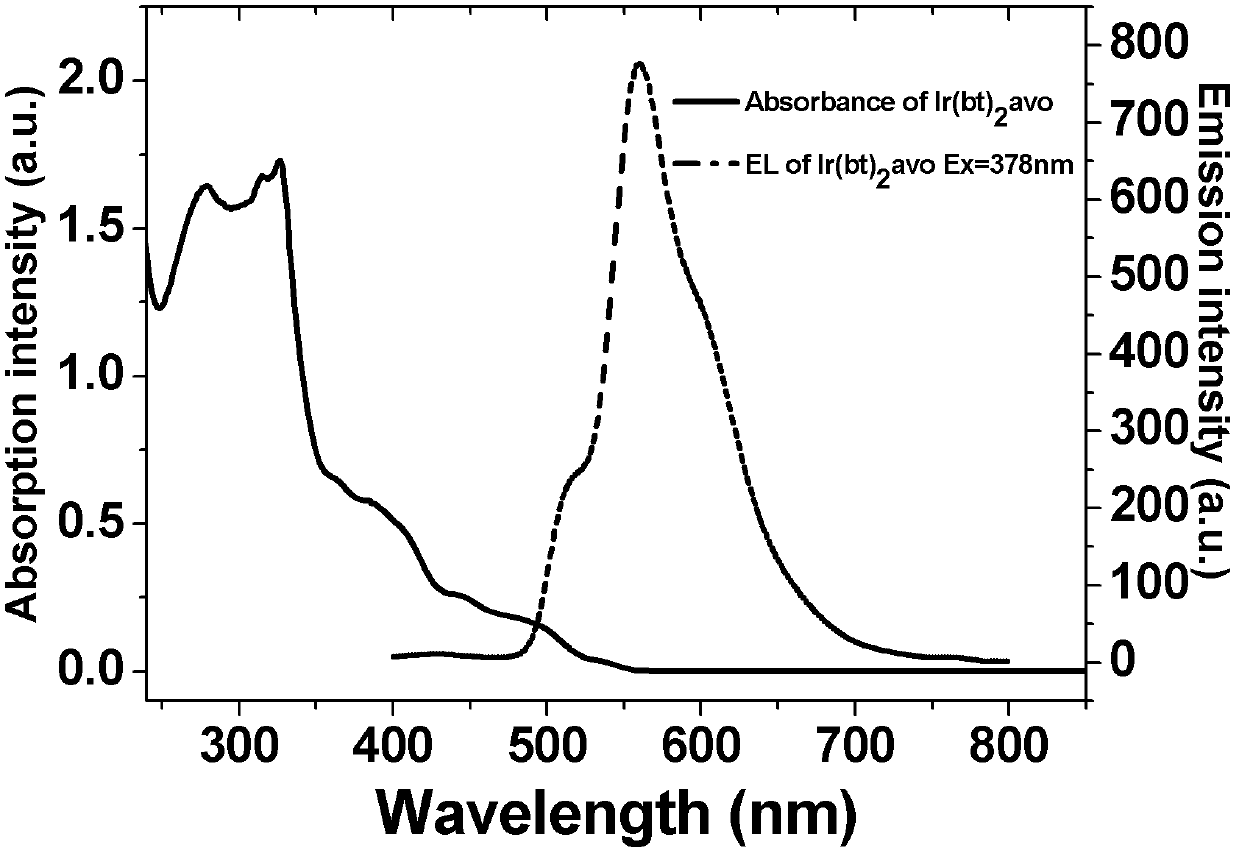
[0028] Example 2 Weigh 648 mg [Ir(bt) 2 Cl] 2 , 341 mg of avobenzone and 230 mg of anhydrous sodium carbonate were added to 50 mL of ethylene glycol nitrogen ethyl ether, under nitrogen protection, refluxed for 24 hours, allowed to stand at room temperature, the solvent was spin-dried, and dichloromethane : Petroleum ether=2:1 to carry out column chromatography separation and purification to obtain 800 mg target product. Yield: 87%. The product is characterized by NMR, and the results are as follows: 1 H NMR in CDCl 3 , 8.10(m, 2H), 7.90(m, 2H), 7.80(m, 6H), 7.31(m, 4H), 7.19(m, 2H), 6.90(m, 2H), 6.79( m, 2H), 6.68 (m, 2H), 6.52 (m, 2H), 6.41(s, 1H), 3.79(s, 3H), 1.28 (s, 9H). MS (EI): m / z 922 (M + ). The spectral property test result of this product is as follows figure 2 shown.
Embodiment 3
[0029] Example 3 Weigh 648 mg [Ir(btp) 2 Cl] 2, 341 mg of avobenzone and 230 mg of anhydrous sodium carbonate were added to 50 mL of ethylene glycol nitrogen ethyl ether, under nitrogen protection, refluxed for 24 hours, allowed to stand at room temperature, the solvent was spin-dried, and dichloromethane : Petroleum ether=2:1 to carry out column chromatography separation and purification to obtain 780 mg target product. Yield: 84%. MS (EI): m / z 922 (M + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com