Non-ferrous metal oxide ore chelate collector and preparation method thereof
A technology of non-ferrous metals and collectors, applied in the field of beneficiation reagents, can solve the problems of low flotation index, low flotation index of reagent dosage, and inability to be applied on a large scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
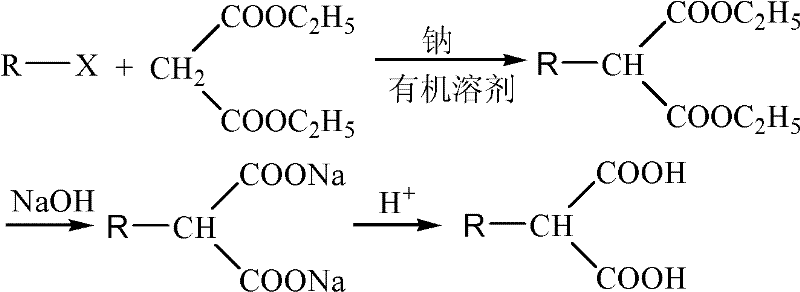
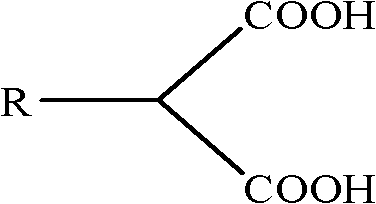
[0031] Add 0.3mol diethyl malonate, 0.36mol benzyl chloride, 0.33mol anhydrous potassium carbonate, and 0.003mol cetyltrimethylammonium bromide successively in the three-necked flask, and start heating to 120°C. After reacting under this stable condition for 2.0 hours, the stirring was stopped to terminate the reaction. After cooling, anhydrous potassium carbonate was removed by filtration, and unreacted benzyl chloride was distilled off under reduced pressure to obtain diethyl benzylmalonate with a yield of 88.7%. At room temperature, add diethyl benzylmalonate dropwise into a 40% NaOH aqueous solution under stirring. During the dropwise addition, a white powder substance is gradually precipitated. After the dropwise addition, continue to react for 2.0 hours, then stop stirring , and filtered to obtain white disodium benzyl malonate. Dissolve benzylmalonate disodium salt in 50mL of water by heating, add hydrochloric acid to acidify to pH=1.0 or so. The white powdery benzyl...
Embodiment 2
[0034] Add 0.3 mol of diethyl malonate, 0.36 mol of bromooctane, 0.33 mol of anhydrous sodium carbonate, and 0.003 mol of polyethylene glycol 400 into the three-necked flask in sequence, and start heating to 100°C while stirring. After reacting under this stable condition for 2.0 hours, the stirring was stopped to terminate the reaction. After cooling, anhydrous potassium carbonate was removed by filtration, and unreacted bromooctane was distilled off under reduced pressure to obtain diethyl octylmalonate with a yield of 91.3%. At room temperature, add diethyl octylmalonate dropwise into a stirred 40% NaOH aqueous solution. During the dropwise addition, a white powder substance is gradually precipitated. After the dropwise addition, continue to react for 1.0 hour, then stop stirring , and filtered to obtain white disodium octyl malonate. Dissolve octylmalonate disodium salt in 50mL of water by heating, add hydrochloric acid to acidify to about pH=1.0-2.0. Obtained octylmalon...
Embodiment 3
[0037]Add 0.3 mol of diethyl malonate, 0.42 mol of n-bromododecane, 0.33 mol of anhydrous sodium carbonate, and 0.015 mol of tetrabutylammonium bromide into the three-necked flask, and start heating to 140°C under stirring . After reacting under this stable condition for 2.0 hours, the stirring was stopped to terminate the reaction. After cooling, anhydrous potassium carbonate was removed by filtration, and unreacted dodecane bromide was distilled off under reduced pressure to obtain diethyl dodecylmalonate with a yield of 87.3%. At room temperature, add diethyl n-dodecylmalonate dropwise into a stirred 40% NaOH aqueous solution. During the dropwise addition, a white powder substance is gradually precipitated. After the dropwise addition, continue to react for 1.0 hour , stop stirring, and filter to obtain white n-dodecylmalonate disodium salt. Dissolve n-dodecylmalonate disodium salt in 50 mL of water by heating, add hydrochloric acid to acidify to about pH=1.0-2.0. The wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com