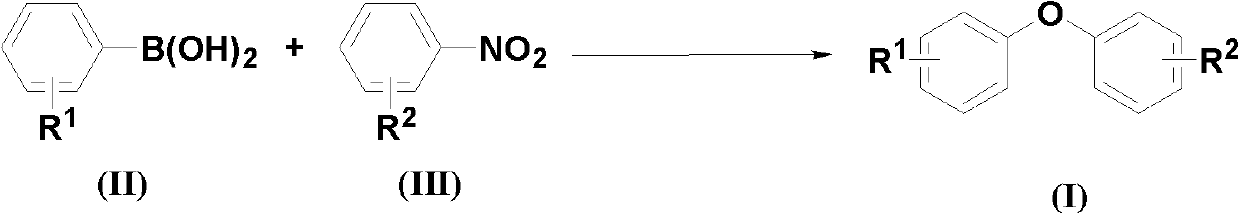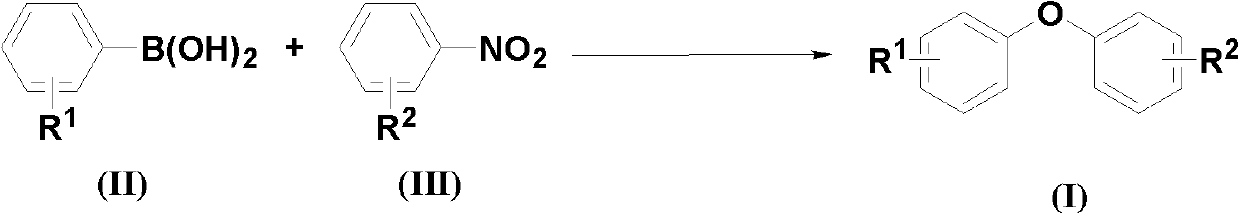Method for synthesizing asymmetric diaryl ether derivative
A technology of diaryl ether and synthesis method, applied in ether preparation, organic chemistry and other directions, to achieve the effects of mild reaction conditions, good product quality, advanced and reasonable process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] According to the molar ratio of aryl boric acid, nitrobenzene compound, copper catalyst, additive, and basic compound, feeding is 1.0:2.0:0.05:1.0:3.0; aryl boric acid is phenylboric acid, and the feeding quality is 12.2g (0.1mol); The base benzene compound is p-nitrobenzaldehyde, and the feeding quality is 30.2g (0.2mol); the copper catalyst is copper oxide, and the feeding quality is 0.40g (0.005mol); the additive is potassium hydrogen persulfate compound salt, and the feeding quality is 61.5g (0.1 mol); the basic compound is cesium carbonate, and the feeding quality is 97.7g (0.3mol); the organic solvent is N,N-dimethylformamide 183g, and its total consumption is 15 times of the quality of phenylboronic acid.
[0028] Put phenylboronic acid, p-nitrobenzaldehyde, copper oxide, potassium persulfate compound salt, and cesium carbonate into the reaction kettle, add N,N-dimethylformamide to dissolve, the reaction temperature is 100°C, and react after 48 hours Finish.
[...
Embodiment 2
[0032] According to phenylboronic acid, p-nitrobenzaldehyde, copper catalyst, potassium persulfate compound salt, cesium carbonate molar ratio is 1.0: 2.0: 0.05: 1.0: 3.0 feeding, phenylboric acid 12.2g (0.1mol); p-nitrobenzene 30.2g (0.2mol) of formaldehyde; 1.0g (0.005mol) of copper acetate; 61.5g (0.1mol) of potassium hydrogen persulfate compound salt; 97.7g (0.3mol) of cesium carbonate; the organic solvent is N,N-dimethylformaldehyde Amide 183g, its total consumption is 15 times of the quality of phenylboronic acid.
[0033] All the other are the same as in Example 1, 16.6g of the resulting product 4-phenoxybenzaldehyde, with a yield of 84% and a purity of 98.3%.
Embodiment 3
[0035] According to phenylboronic acid, p-nitrobenzaldehyde, copper catalyst, potassium persulfate compound salt, cesium carbonate molar ratio is 1.0: 2.0: 0.05: 1.0: 3.0 feeding, phenylboric acid 12.2g (0.1mol); p-nitrobenzene 30.2g (0.2mol) of formaldehyde; 1.8g (0.005mol) of copper trifluoromethanesulfonate; 61.5g (0.1mol) of potassium persulfate compound salt; 97.7g (0.3mol) of cesium carbonate; the organic solvent is N, N- Dimethylformamide 183g, its total consumption is 15 times of the quality of phenylboronic acid.
[0036] The rest are the same as in Example 1, 9.7 g of the resulting product 4-phenoxybenzaldehyde, a yield of 49%, and a purity of 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com