Method for in-vitro screening of PXR activation characteristics
A characteristic and carrier technology, applied in the field of in vitro PXR activation characteristic screening, to achieve the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0027] 1 Materials and methods
[0028] 1.1 Materials
[0029] HepG2 cell line was purchased from Xiehe Cell Center, MEM medium, non-essential amino acid (NEAA), fetal bovine serum were purchased from Hyclone Company, rifampicin was purchased from Merck Company, T4 ligase, pGLuc-Basic reporter gene plasmid, restriction endonuclease Dicer and luciferase activity assay kits were purchased from NEB Company, transfection reagent Lipofectamine TM LTX and G418 were purchased from Invitrogen Company, plasmid medium extraction kit, mini-extraction kit and gel recovery kit were purchased from Promega Company, Probest Taq enzyme system was purchased from TaKaRa Company, and the constructed pGL3-3A4 dual luciferase reporter gene plasmid, The hPXR expression vector was provided by Zhejiang University.
[0030] 1.2 Method
[0031]1.2.1 Construction of the reporter gene vector: design primers (see Table 1 for the sequence), use the pGL3-3A4 dual luciferase reporter gene plasmid as a tem...
Embodiment 1
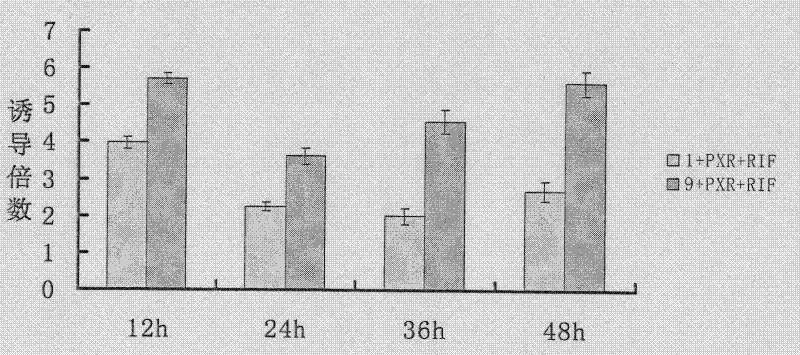
[0054] Example 1: Method for Screening PXR Activation Properties in Vitro
[0055] 1), the construction of reporter gene carrier:
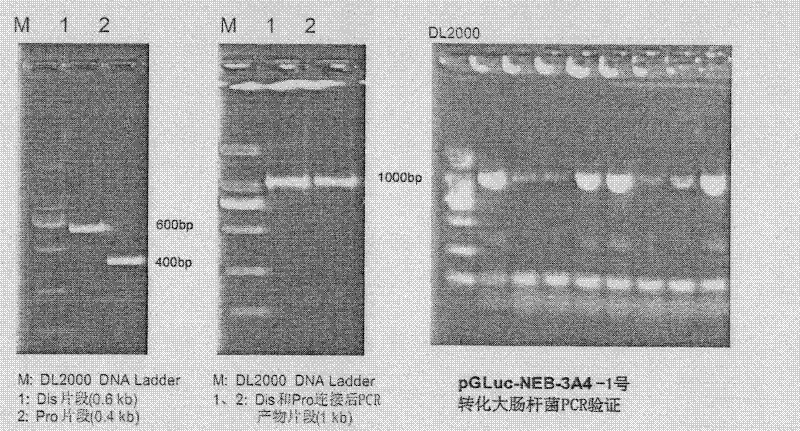
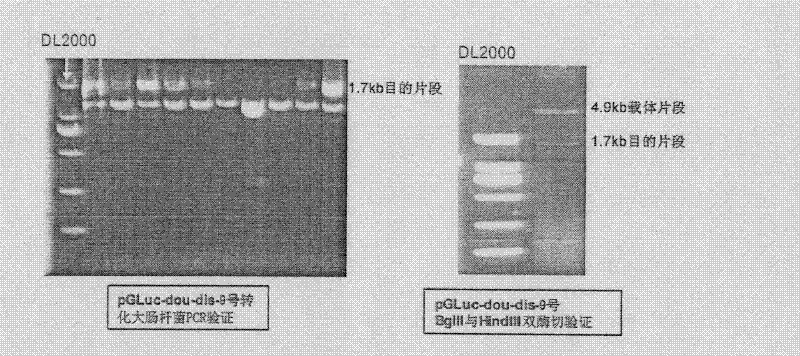
[0056] Primers were designed, using the pGL3-3A4 dual-luciferase reporter gene plasmid as a template, and the primer pairs dis-F / dis-R and pro-F / pro-R were used to amplify the remote regulatory sequence of the CYP3A4 promoter (-7833~- 7208) and the proximal promoter sequence (-361 ~ +11), the distal regulatory sequence and the proximal regulatory sequence were subjected to EcoR I-EcoR V and EcoR V-Hind III double enzyme digestion, and the two double The digested products were ligated, and then the primer pair dis-F / pro-R was used to clone the linear tandem fragments of the distal regulatory sequence and the proximal regulatory sequence, which were connected to the EcoR I / HindIII site of the pGLuc-Basic vector, and transformed into Escherichia coli , verified by enzyme digestion and sequencing, the vector was named pGLuc-NEB-3A4-1, that is, No. 1; t...
Embodiment 2
[0062] Embodiment 2: A method for screening the activation characteristics of PXR in vitro, the method comprising the following steps:
[0063] 1), the construction of reporter gene carrier:
[0064] Primers were designed, using the pGL3-3A4 dual-luciferase reporter gene plasmid as a template, and the primer pairs dis-F / dis-R and pro-F / pro-R were used to amplify the remote regulatory sequence of the CYP3A4 promoter (-7833~- 7208) and the proximal promoter sequence (-361 ~ +11), the distal regulatory sequence and the proximal regulatory sequence were subjected to EcoR I-EcoR V and EcoR V-Hind III double enzyme digestion, and the two double The digested products were ligated, and then the primer pair dis-F / pro-R was used to clone the linear tandem fragments of the distal regulatory sequence and the proximal regulatory sequence, which were connected to the EcoR I / Hind III site of the pGLuc-Basic vector, and transformed into large intestine Bacillus, verified by enzyme digestion ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com