Synthesis method for N-Methyl-o-Phenylenediamine (salt) and isomeride thereof
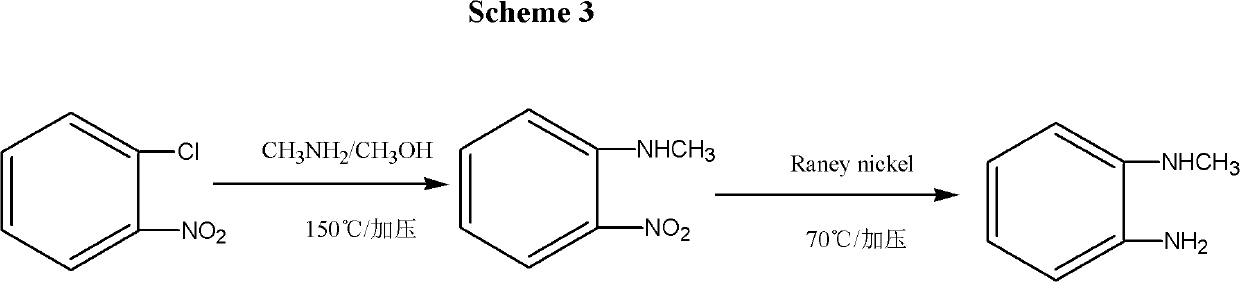
A technology of methyl-o-phenylenediamine and isomers, which is applied in the field of telmisartan intermediates of sartans for treating hypertension, can solve the problem of increasing the production cost of the intermediates and the expensive price of o-nitrochlorobenzene , product quality is difficult to guarantee and other problems, to achieve the effect of simple reaction, strong selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
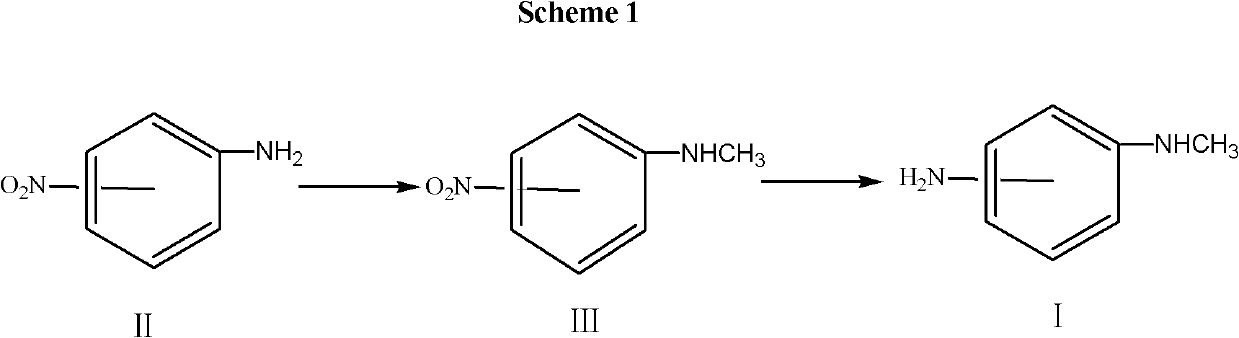
[0038] 1. Synthesis of N-methyl o-nitroaniline
[0039] Mix 40.0g, 0.29mol of o-nitroaniline with 200ml of acetone, then add 32g, 0.57mol of KOH, dropwise add 46g, 0.37mol of methylating reagent dimethyl sulfate, after monitoring the point of no raw material by TLC, add 20.0ml of ammonia water, and Press the acetone back to dryness, then add 200.0ml of water, stir and crystallize, separate by filtration, and dry to obtain 42.0g of N-methyl-o-nitroaniline, with a yield of 95.3%. Purity >99%.
[0040] 2. Synthesis of N-methyl o-phenylenediamine
[0041] Mix 20.0g, 0.13mol of N-methyl o-nitroaniline with 100ml of methanol, then add 0.05g of 10% Pd / C into the hydrogenation reactor, and feed hydrogen at 30-35°C under 0.2-0.5MPa about 3 hours. After filtration, 17.8 g of thionyl chloride, 0.15 mol, was added dropwise to the filtrate, and the temperature was lowered for filtration to obtain 25 g of N-methyl o-phenylenediamine dihydrochloride, with a yield of 98.4%.
Embodiment II
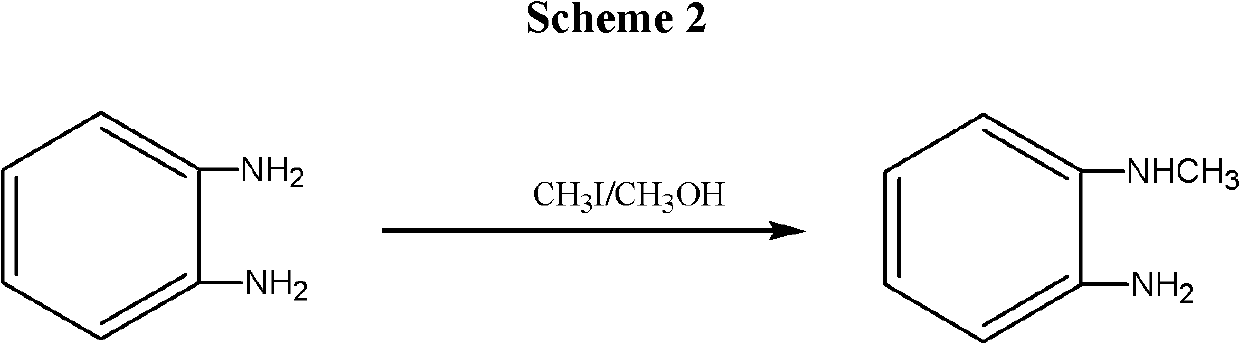
[0043] 1. Synthesis of N-methyl o-nitroaniline
[0044] Mix 40.0g, 0.29mol of o-nitroaniline and 200ml of N,N-dimethylformamide, then add 22.8g, 0.57mol of NaOH, dropwise add 52.5g, 0.37mol of methyl iodide reagent, and monitor by TLC After there is no raw material point, add 200.0 ml of water, stir and crystallize, filter and separate, and dry to obtain 42.0 g of N-methyl-o-nitroaniline, with a yield of 95.3%. Purity >99%.
[0045] 2. Synthesis of N-methyl o-phenylenediamine
[0046] Add 100ml of ethanol, 5ml of glacial acetic acid, 21.8g of reduced iron powder, 0.39mol into a 500ml reaction bottle, stir and activate at 50-55°C for 30 minutes, then add 20.0g of N-methyl o-nitroaniline, 0.13mol and heat up to reflux reaction about 2 hours. Filtrate while hot, add thionyl chloride 17.8g, 0.15mol dropwise to the filtrate, cool down and filter to obtain 22.8g of N-methyl o-phenylenediamine dihydrochloride, yield 90.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com