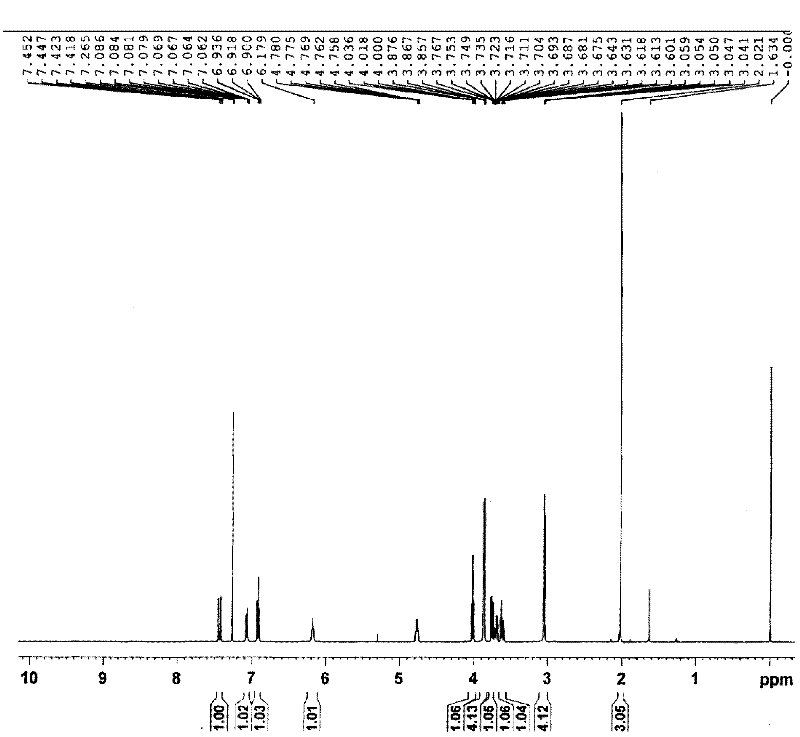
Method for preparing inezolid
A technology of linezolid and acetamide, applied in the field of preparation of linezolid, can solve the problems of harsh reaction conditions, low yield of linezolid, complicated purification operation of intermediates, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of 3-fluoro-4-morpholinonitrobenzene
[0038] At room temperature, morpholine (26ml, 297mmol), N,N-diisopropylethylamine (51.2ml, 293mmol), and ethyl acetate (150ml) were added into a 500ml three-necked flask. 3,4-Difluoronitrobenzene (30ml, 271mmol) was added dropwise. After dropping, stir overnight at room temperature and react for 24 hours. The next day, a TLC plate was used to determine if the reaction was complete. (The solvent system is petroleum ether:ethyl acetate=5:1). Dichloromethane (100ml), ethyl acetate (420ml), water (200ml) were added to the reaction mixture. Two-phase separation, organic phase with anhydrous MgSO 4 Let dry for 2 hours. Remove MgSO by filtration 4 . A yellow solid was obtained by rotary evaporation, and 48.9 g of a solid was obtained by vacuum drying, with a yield of 79.3%.
[0039] Synthesis of 3-Fluoro-4-Morpholinylaniline by Reduction of Hydrazine Hydrate Catalyzed by Ferric Oxyhydroxide
[0040] Step 1: Prepar...
Embodiment 2
[0061] Preparation of 3-fluoro-4-morpholinonitrobenzene
[0062]At room temperature, morpholine (52ml, 594.mmol), N,N-diisopropylethylamine (102.4ml, 586mmol), and ethyl acetate (300ml) were added into a 500ml three-necked flask. 3,4-Difluoronitrobenzene (60ml, 542mmol) was added dropwise. After dropping, stir overnight at room temperature and react for 24 hours. The next day, a TLC plate was used to determine if the reaction was complete. (The solvent system is petroleum ether:ethyl acetate=5:1). Dichloromethane (200ml), ethyl acetate (840.ml), water (400ml) were added to the reaction mixture. Two-phase separation, organic phase with anhydrous MgSO 4 Let dry for 2 hours. Remove MgSO by filtration 4 . A yellow solid was obtained by rotary evaporation, and 48.9 g of a solid was obtained by vacuum drying, with a yield of 79.3%.
[0063] Synthesis of 3-Fluoro-4-Morpholinylaniline by Reduction of Hydrazine Hydrate Catalyzed by Ferric Oxyhydroxide
[0064] Step 1: Prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com