6-amine-substituted-6-deoxidizing chitosan derivant and preparation method thereof
A technology of deoxychitosan and derivatives, applied in the field of marine chemical engineering, can solve the problems of poor solubility, limited use, weak bacteriostatic activity, etc., and achieve good solubility, enhanced bacteriostatic effect, and strong regional selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] Preparation method of derivatives: chitosan in dimethylformamide (DMF) and phthalic anhydride to protect the 2-position NH 2 Under the premise of carrying out 6-position bromination reaction, the reaction temperature is 100-130 ℃, and the reaction time is 5-10 hours; after the reaction, the product is poured into ice water, filtered, and dried after washing the filter cake with sufficient water and ethanol, Obtained N-phthaloyl chitosan. The mass-volume ratio of chitosan and DMF is 1:20-100, and the reaction molar ratio of chitosan and phthalic anhydride is 1:3-5. The above N-phthaloyl chitosan derivatives were dispersed in N-methylpyrrolidone (NMP), and N-bromosuccinimide (NBS) and triphenylene were added in an ice-water bath and under nitrogen protection. Phosphine (TPP), followed by reaction at 50-100°C for 1-4 hours. The obtained product was precipitated with ethanol, the supernatant was discarded by centrifugation, washed repeatedly with ethanol, and finally drie...
Embodiment 1
[0034] Preparation of Example 1 Derivative 1
[0035]Put 1.0 g of 6-bromo-6-deoxy-N-phthaloyl chitosan and 26 ml of ethylenediamine into a three-necked flask, react at 80°C for 24 hours, pour the reactant into 50 ml of isopropanol, and the resulting precipitate Repeated washing with ethanol, centrifugation and freeze-drying to obtain an intermediate. Take 0.5g of the intermediate and add it to the three-necked flask, then add 25ml of NMP and 25ml of hydrazine hydrate, and react at 100°C for 4 hours. The reaction product is precipitated with ethanol, washed repeatedly by centrifugation, and freeze-dried to obtain derivative 1. The structural formula is shown in Table 1.
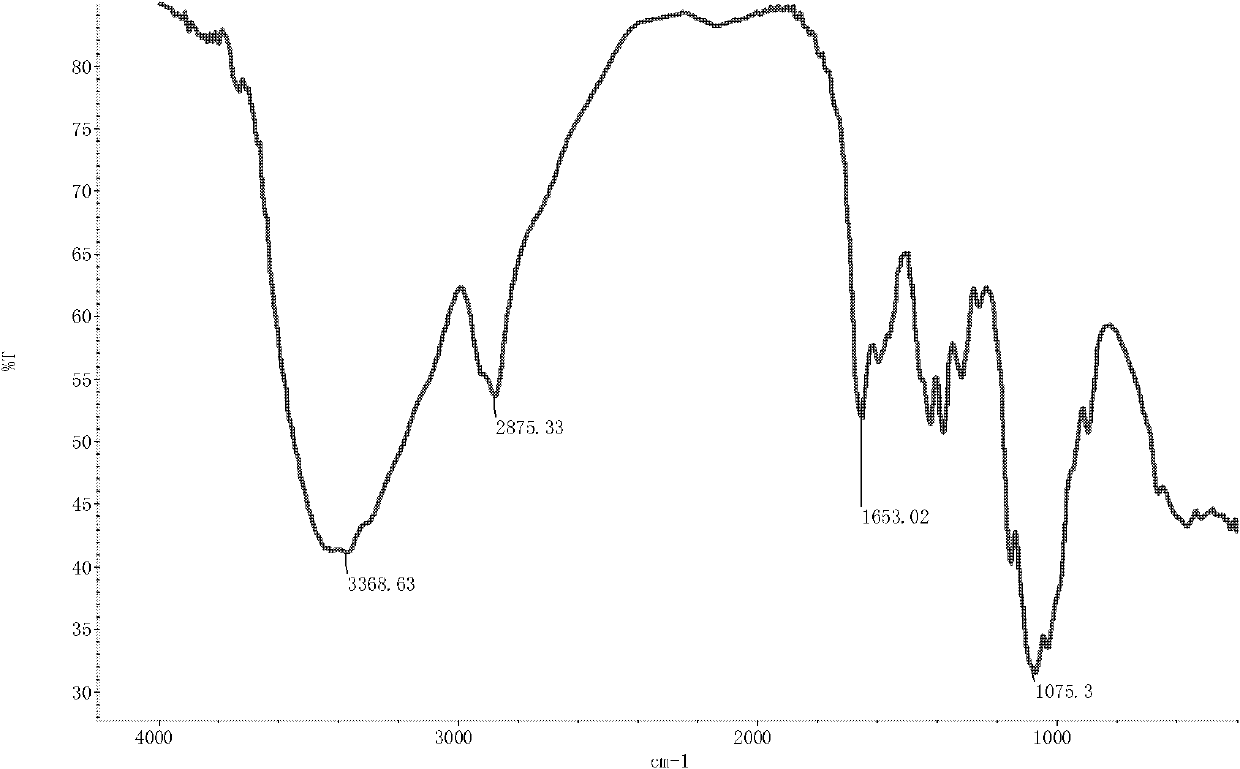
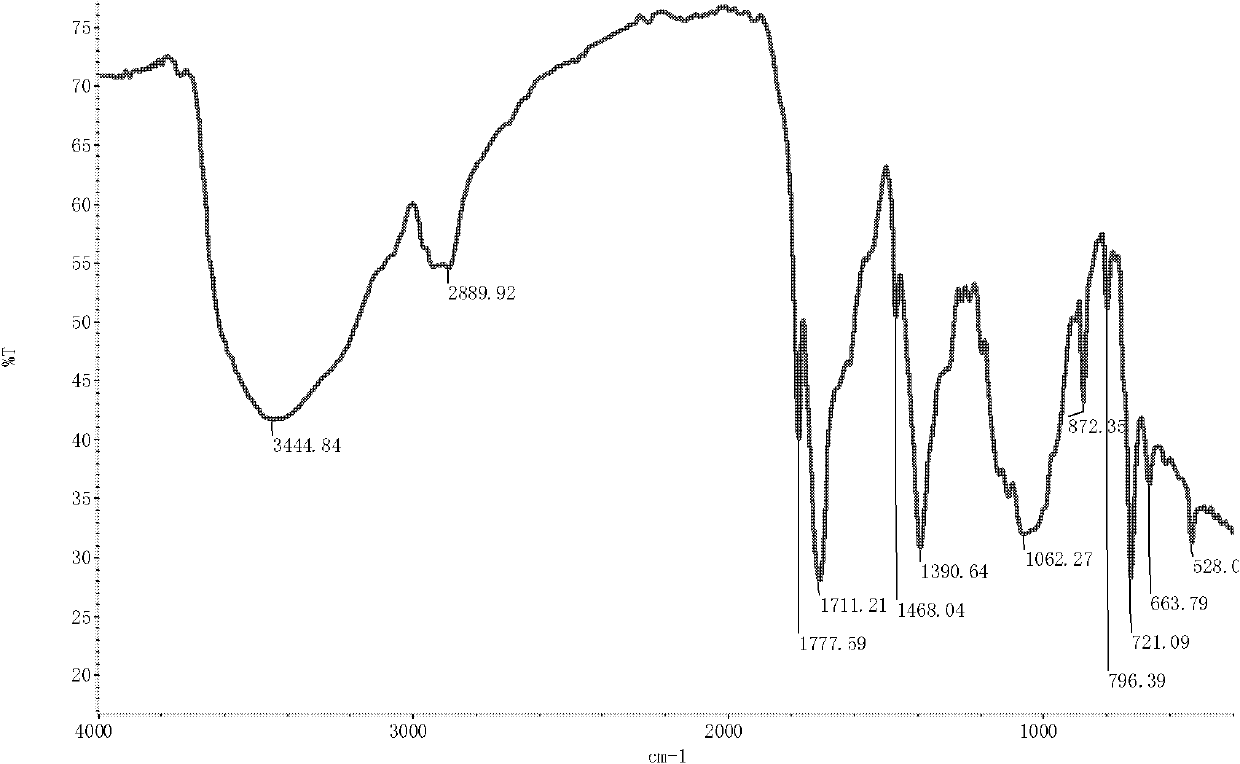
[0036] The infrared spectrum shows that: the infrared spectrum of chitosan derivative 1 ( image 3 ) and the infrared spectra of chitosan ( figure 1 ), the N-H stretching vibration peak of the primary amine group is broadened, making 2889.92cm -1 The C-H stretching vibration peak at 1583.70cm becomes the sh...
Embodiment 2
[0037] Example 2 Preparation of Derivative 2
[0038] Put 1.0 g of 6-bromo-6-deoxy-N-phthaloyl chitosan, 26 ml of diethylenetriamine, and 10 ml of distilled water into a three-necked flask, react at 80°C for 48 hours, and pour the reactant into 50 ml of isopropanol , the obtained precipitate was repeatedly washed with ethanol, centrifuged and freeze-dried to obtain an intermediate. Take 0.5g of the intermediate and add it to the three-necked flask, then add 25ml of NMP and 25ml of hydrazine hydrate, and react at 100° C. for 4 hours. The reaction product is precipitated with ethanol, washed repeatedly by centrifugation, and freeze-dried to obtain derivative 2. The structural formula is shown in Table 1.
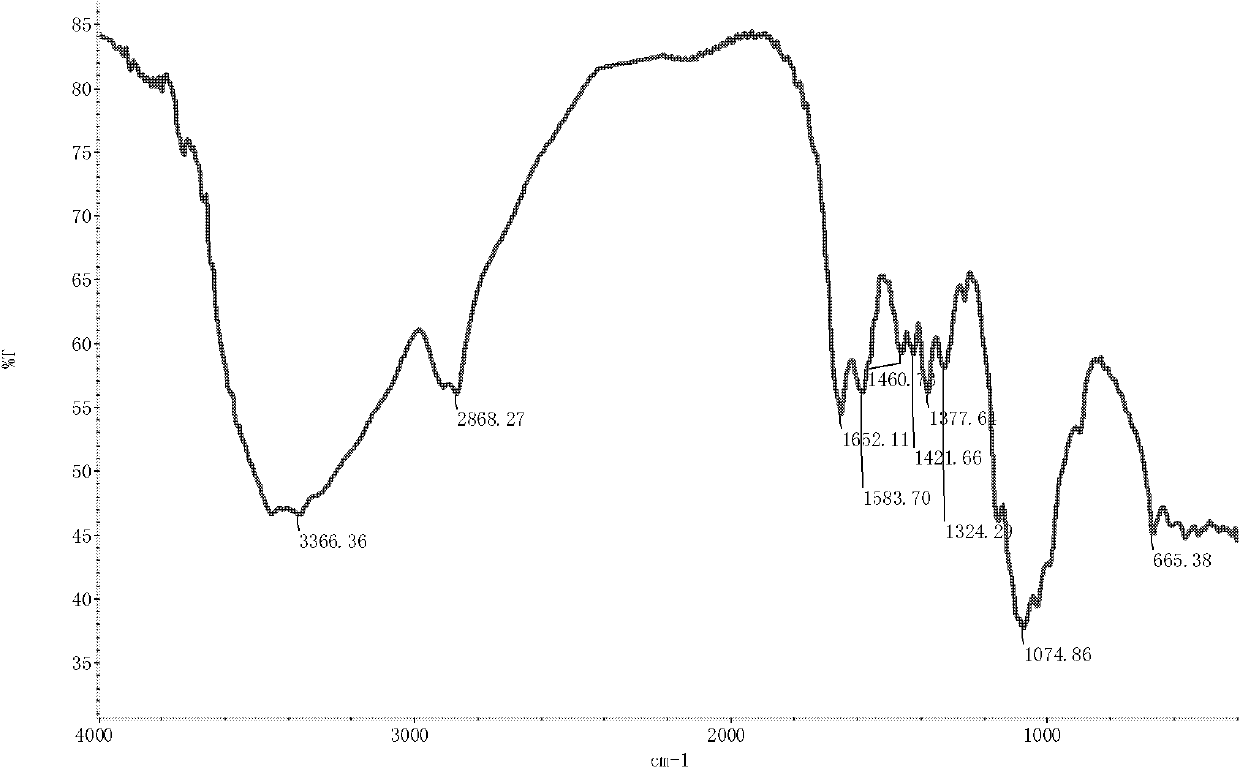
[0039] The infrared spectrum shows: the infrared spectrum of chitosan derivative 2 ( Figure 4 ) and the infrared spectra of chitosan ( figure 1 ) compared to 3425.93 and 2872.43cm -1 The signal of N-H stretching vibration peak and hydrocarbon stretching vibration peak of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com