Aztreonam composition freeze-dried powder for injection
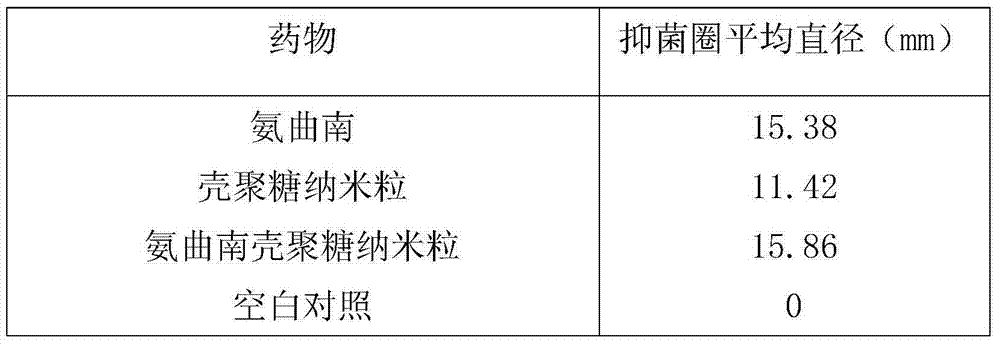
A technology of freeze-dried powder injection and aztreonam, which is applied in the direction of freeze-dried delivery, powder delivery, medical preparations of non-active ingredients, etc., and can solve the problem of no dosage form containing chitosan nanoparticles, large amount of use, and difficulty in mixing Uniformity and other issues, to achieve the effect that is beneficial to clinical application, eliminate active effect, and enhance antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1. Preparation of aztreonam composition freeze-dried powder for injection, in 1000 vials.
[0031] prescription:
[0032] Aztreonam 250g
[0033] Chitosan Nanoparticles 100g
[0034] Water for injection 2000ml
[0035] 2. Preparation process:
[0036] (1) Weigh 250g of chitosan nanoparticles and slowly add them to 2000ml of water for injection, and stir until dissolved while adding.
[0037] (2) Add 500g of aztreonam and stir to dissolve until clear.
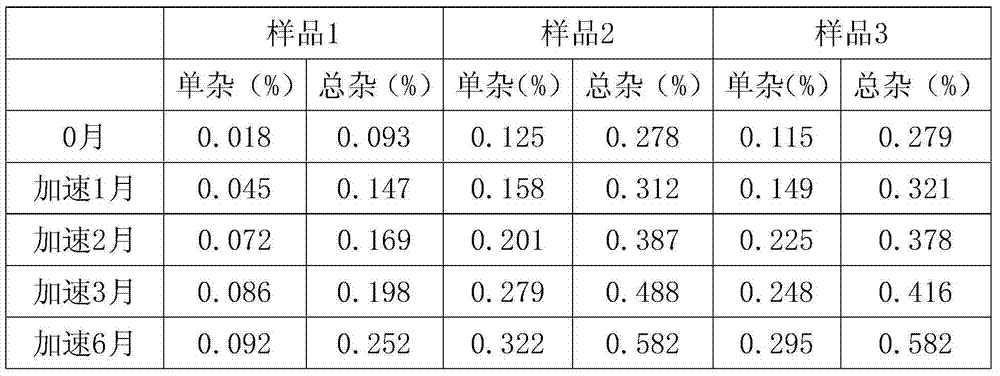
[0038] (3) Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect The intermediate content is calculated as 0.5g per bottle based on aztreonam.
[0039] (4) Fill according to the testing requirements, put it into a freeze dryer after half-tamping, cool down to -40°C, keep warm for 2 hours, slowl...
Embodiment 2
[0040] Example 2: Preparation of aztreonam composition freeze-dried powder for injection, in 1000 vials.
[0041] 1. Prescription:
[0042] Aztreonam 250g
[0043] Chitosan Nanoparticles 125g
[0044] Water for injection 2000ml
[0045] 2. Preparation process:
[0046] (1) Weigh 250g of chitosan nanoparticles and slowly add them to 2000ml of water for injection, and stir until dissolved while adding.
[0047] (2) Add 500g of aztreonam and stir to dissolve until clear.
[0048] (3) Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect The intermediate content is calculated as 0.5g per bottle based on aztreonam.
[0049] (4) Fill according to the testing requirements, put it into a freeze dryer after half-tamping, cool down to -40°C, keep warm for 2 hours, slowl...
Embodiment 3
[0050] Example 3: Preparation of aztreonam composition freeze-dried powder for injection, in 1000 vials.
[0051] prescription:
[0052] Aztreonam 250g
[0053] Chitosan Nanoparticles 150g
[0054] Water for injection 2000ml
[0055] 2. Preparation process:
[0056] (1) Weigh 300g of chitosan nanoparticles and slowly add to 2000ml of water for injection, stir until dissolved while adding.
[0057] (2) Add 500g of aztreonam and stir to dissolve until clear.
[0058] (3) Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect The intermediate content is calculated as 0.5g per bottle based on aztreonam.
[0059] (4) Fill according to the inspection requirements, and send it into the freeze dryer after half-tamping, and leave the box after freeze-drying, with good for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com