Method for separating and determining methyhaaltrexone bromide and impurities thereof with liquid chromatography
A technology of methylnaltrexone bromide and liquid chromatography, which is applied in the direction of material separation, measuring devices, and analysis materials, can solve problems such as the inability to separate methylnaltrexone bromide, and achieve low cost, easy preparation, and accurate determination of impurities Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Instruments and Conditions
[0047] Dianne Ultimate 3000 high-performance liquid chromatography and chemical workstation; automatic sample injection; with Breeze AQ-C18 (4.6 × 250mm, 5μm) as the chromatographic column; set the wavelength of the ultraviolet detector to 310nm; mobile phase: 0.1% trifluoroacetic acid Solution is mobile phase A, with methanol as mobile phase B, carry out gradient elution in the following manner:: 0 minute to 10 minutes, mobile phase A is 85% (V / V), mobile phase B is 15% (V / V V); 10 minutes to 35 minutes, mobile phase A linearly decreased to 60% (V / V), and mobile phase B linearly increased to 40% (V / V); 35 minutes to 45 minutes, mobile phase A was 60% ( V / V), mobile phase B is 40% (V / V); 45.1 minutes to 55 minutes, mobile phase A is 85% (V / V), mobile phase B is 15% (V / V), that is, 45.1 minutes followed by the equilibrated column. The column temperature was 30° C., the flow rate was 1.0 ml / min, and the injection volume was 20 μl.
[0048] ...
Embodiment 2
[0056] Determination of impurities in methylnaltrexone bromide injection by liquid chromatography.
[0057] Instruments and Conditions
[0058] Agilent 1200 high-performance liquid chromatography and chemical workstation; automatic sampling; chromatographic conditions are the same as in Example 1.
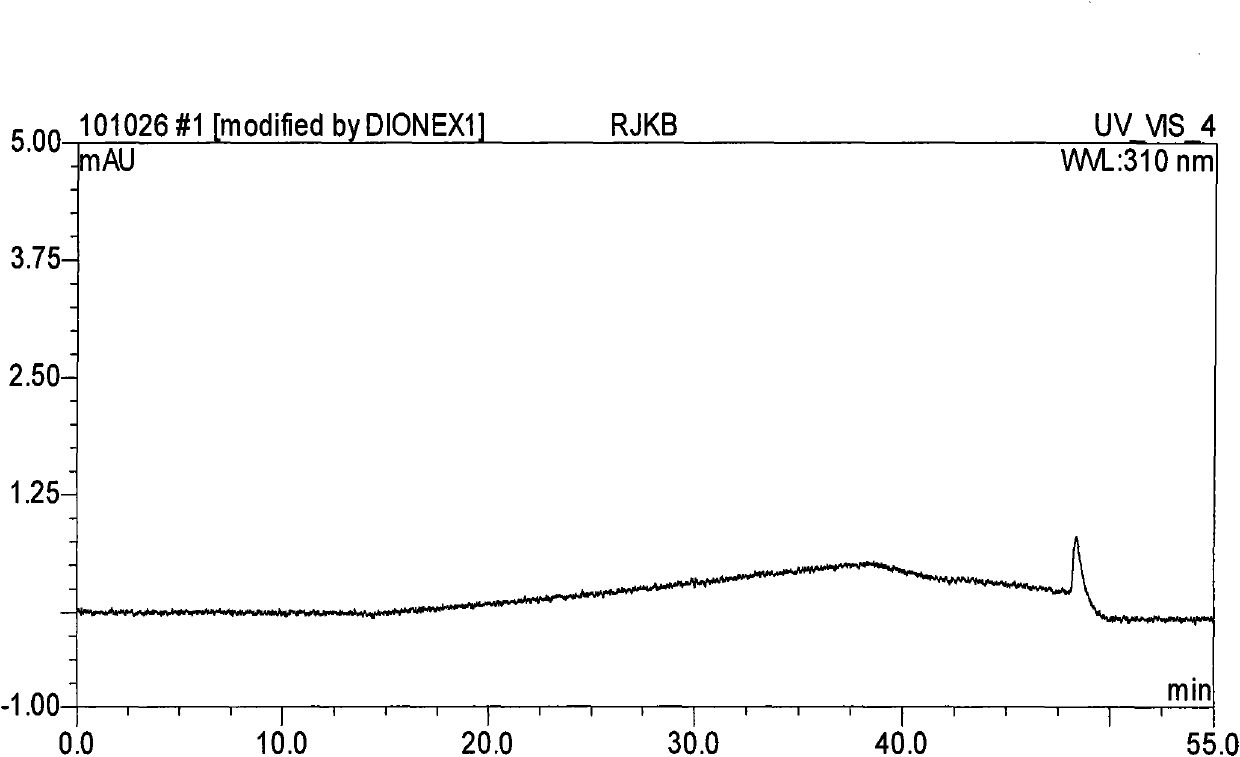
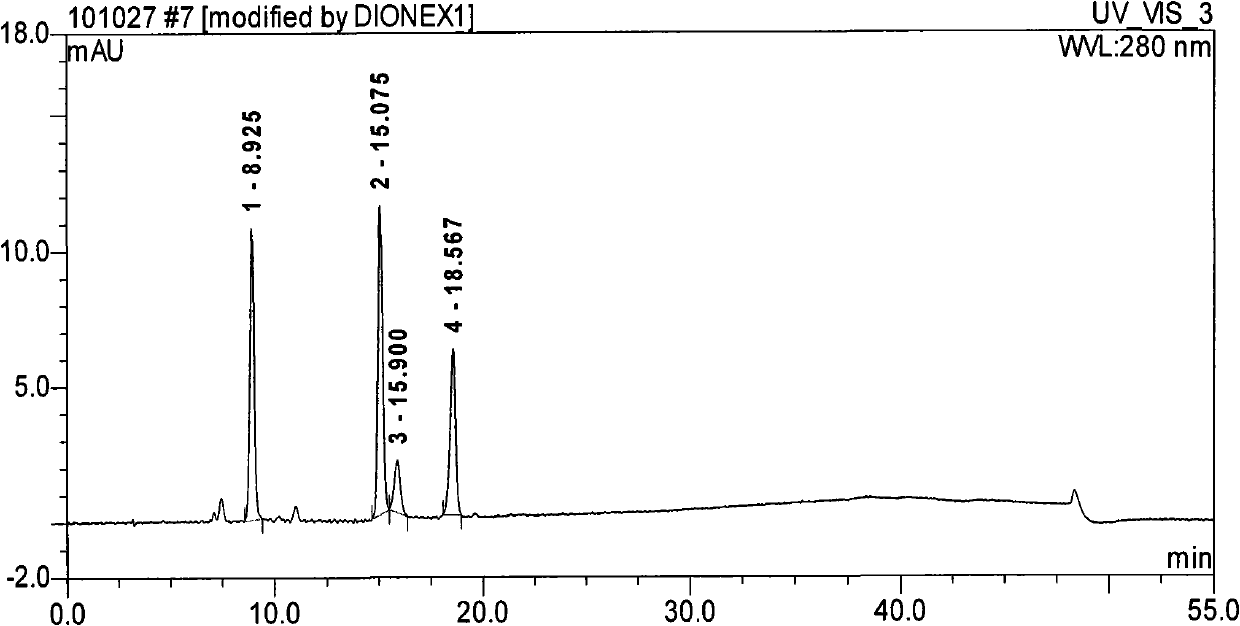
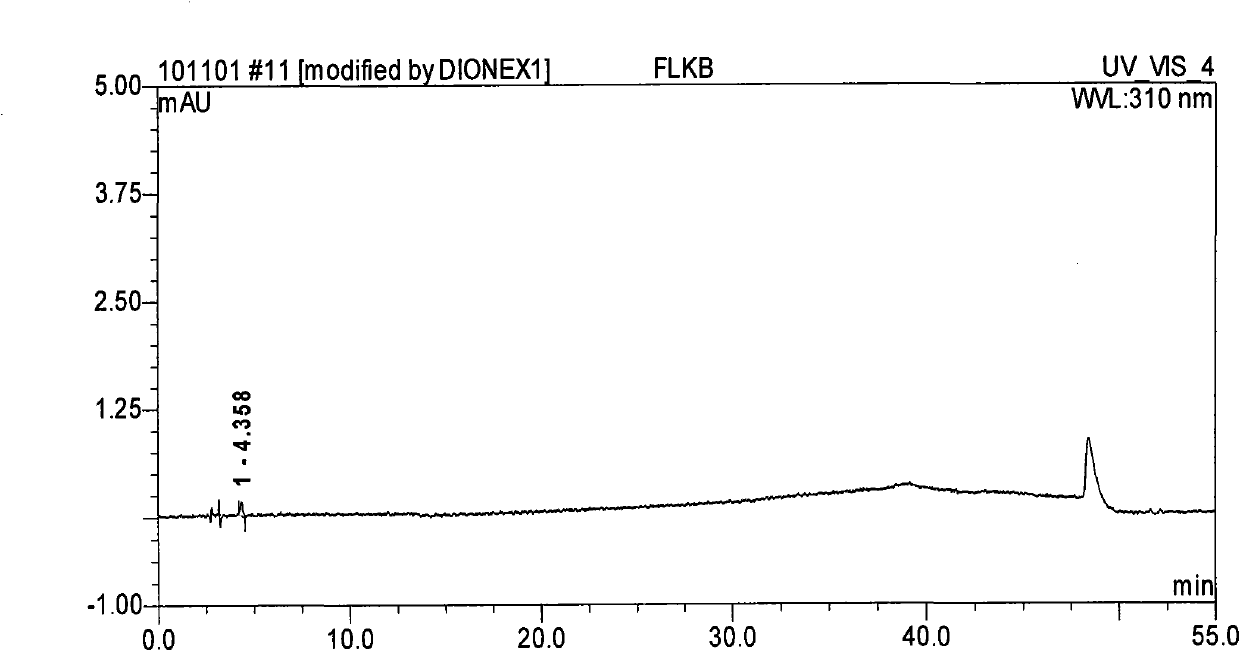
[0059] Take an appropriate amount of methylnaltrexone bromide injection (approximately equivalent to 10 mg of methylnaltrexone bromide), put it in a 10ml measuring bottle, add diluent (mobile phase A: mobile phase B=85:15) to dissolve and dilute to the mark, Shake up, as need testing solution; Precision measures 1.0ml, puts in 10ml measuring bottle, adds diluent and dilutes to scale, shakes up, as contrast solution; Carry out liquid chromatography analysis according to the chromatographic condition of embodiment 2, the results see Figure 5 , Image 6 . Figure 5 , Image 6 It shows that there is almost no impurity detected in the methylnaltrexone bromide bulk drug, indicating ...
Embodiment 3
[0061] Determination of Impurities in Brommethylnaltrexone APIs by Liquid Chromatography
[0062] Instruments and Conditions
[0063] Agilent 1200 high-performance liquid chromatography and chemical workstation; automatic sampling; chromatographic conditions are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com