Cathode material, alpha-Fe2O3, of high-capacity lithium ion battery and preparation method for material
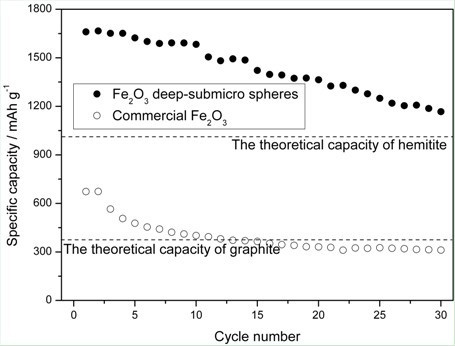
A technology for lithium ion batteries and negative electrode materials, which is applied in the field of electrochemistry, can solve the problem of no reference, and achieve the effects of good cycle stability, cost reduction, and uniform distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
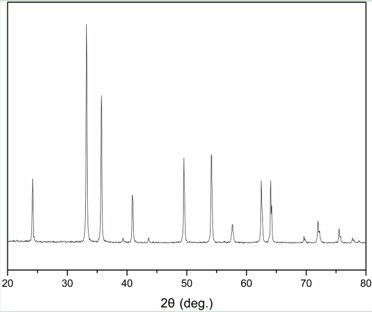
[0045] Weigh 20.2g analytically pure Fe(NO 3 ) 3 9H 2 O was dissolved in deionized water to make 50ml, 1 mol / L Fe(NO 3 ) 3 aqueous solution, and then measure 30ml of 5 mol / L KOH aqueous solution prepared in advance, and pour it into Fe(NO 3 ) 3 Reaction precipitation in aqueous solution to obtain a suspension, and adjust the pH value to 10 with 25% ammonia solution, stir vigorously for half an hour, transfer the mixed solution into a 100ml Teflon hydrothermal reactor, put the reactor into an oven, and keep it at 180°C for 5 hours , cooled to room temperature in the air, separated by filtration, washed, and dried at 80°C for 12 hours to obtain the α - Fe 2 o 3 .
Embodiment 2
[0047] Weigh 20.2g analytically pure Fe(NO 3 ) 3 9H 2 O was dissolved in deionized water to make 50ml, 1 mol / L Fe(NO 3 ) 3 aqueous solution, and then measure 30ml of 5 mol / L KOH aqueous solution prepared in advance, and pour it into Fe(NO 3 ) 3 Reaction precipitation in aqueous solution to obtain a suspension, and adjust the pH value to 10 with 25% ammonia solution, stir vigorously for half an hour, transfer the mixed solution into a 100ml Teflon hydrothermal reactor, put the reactor into an oven, and keep it at 190°C for 5 hours , cooled to room temperature in the air, separated by filtration, washed, and dried at 120°C for 8 hours to obtain the α - Fe 2 o 3 .
Embodiment 3
[0049] Weigh 20.2g analytically pure Fe(NO 3 ) 3 9H 2 O was dissolved in deionized water to make 50ml, 1 mol / L Fe(NO 3 ) 3 aqueous solution, and then measure 30ml of 5 mol / L KOH aqueous solution prepared in advance, and pour it into Fe(NO 3 ) 3 Reaction precipitation in aqueous solution to obtain a suspension, and adjust the pH value to 10 with 25% ammonia solution, stir vigorously for half an hour, transfer the mixed solution into a 100ml Teflon hydrothermal reactor, put the reactor into an oven, and keep it at 200°C for 5 hours , cooled to room temperature in the air, separated by filtration, washed, and dried at 100°C for 12 hours to obtain the α - Fe 2 o 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com