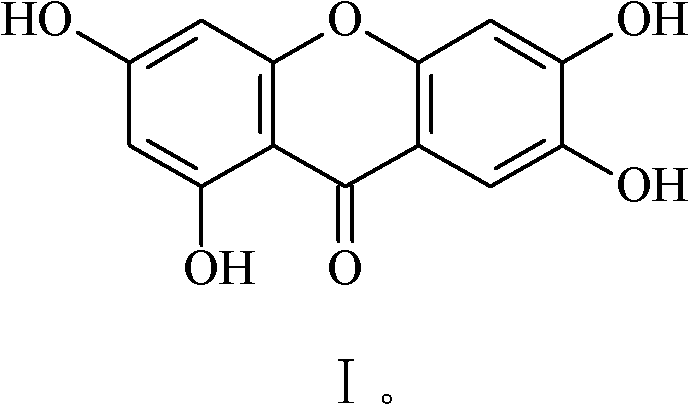
Method for preparing mangiferin aglycone
A technology of mangiferin and compounds, which is applied in the field of medicinal chemistry, can solve the problems that mangiferin cannot be widely used in chemical synthesis, the content of mangiferin and mangiferin is small, and the amount of mangiferin preparation cannot meet the requirements, and the price of raw materials can be achieved. Inexpensive, less synthetic steps, easy to control the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Mangiferin aglycon is prepared by the method of the present invention
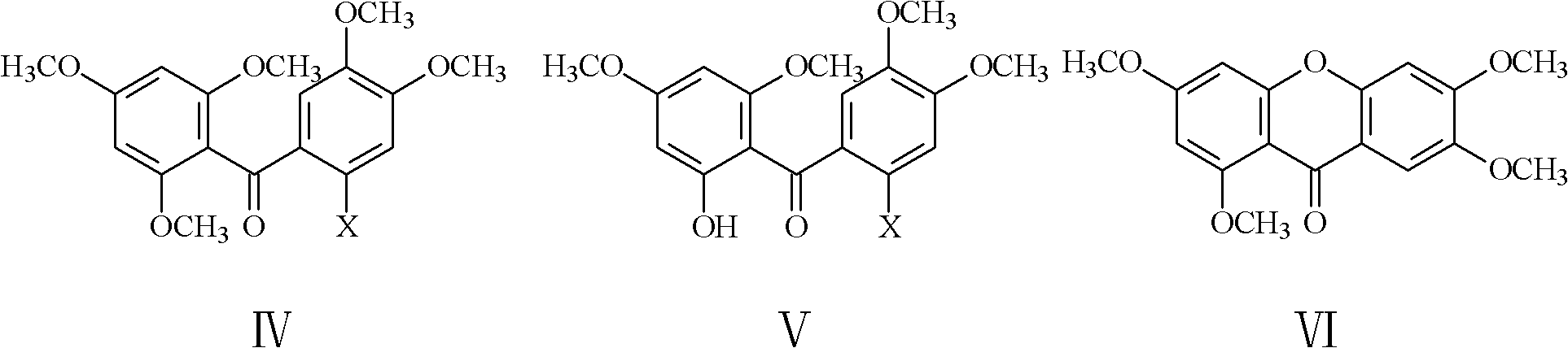
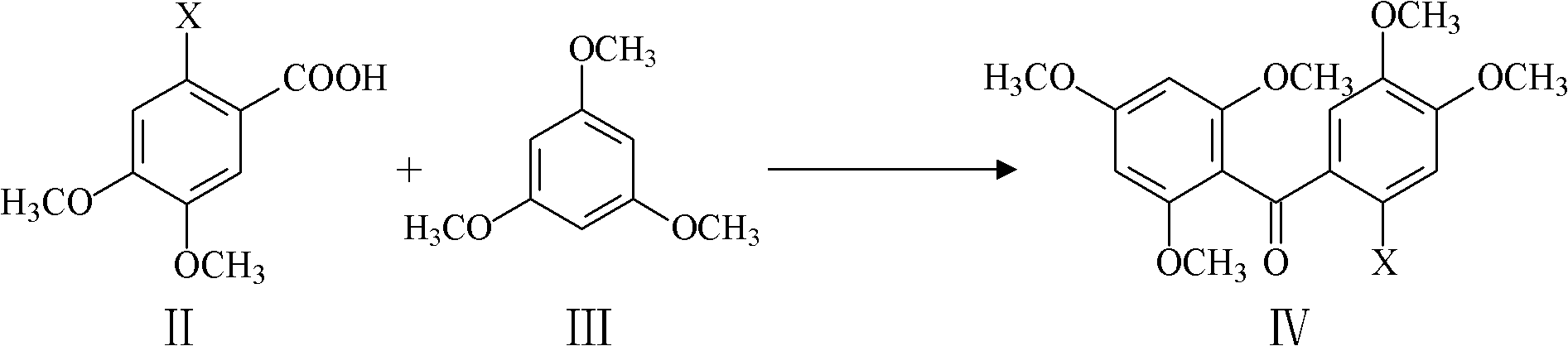
[0044] Weigh 5.04g (0.02mol) of 2-chloro-4,5-dimethoxybenzoic acid and 3.40g (0.02mol) of 1,3,5-trimethoxybenzene into a 100ml three-necked reaction flask, stir 2ml of anhydrous hydrogen fluoride and 20ml of solvent chloroform were added. The mixture was heated to 80°C, stirred and reacted for 10 h, then allowed to cool, added 50 ml of water and 30 ml of chloroform, stirred well and left to stand, separated the organic layer, extracted the water layer once with 50 ml of chloroform, combined the organic layers obtained twice, Washed once with water and semi-saturated aqueous sodium bicarbonate solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to remove the solvent under reduced pressure to obtain a solid, and then recrystallized to obtain 5.7 g of a compound with the structure of formula IV, with a yield of 70%. In formula IV, X is Cl, represented...
Embodiment 2
[0049] Embodiment 2: the method of the present invention prepares mangiferin aglycon
[0050] Weigh 5.22g (0.02mol) of 2-bromo-4,5-dimethoxybenzoic acid and 3.40g (0.02mol) of 1,3,5-trimethoxybenzene into a 100ml three-necked reaction flask, stir 5.0 g of phosphorus pentoxide and 20 ml of solvent trichlorethylene were added. Heat the mixture to 120°C, stir and react for 10 hours, then let it cool, add 50ml of water and 30ml of dichloromethane, stir well and let stand, separate the organic layer, extract the water layer with 50ml of dichloromethane once more, and combine the obtained two times The organic layer was washed once with water and semi-saturated aqueous sodium bicarbonate solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated under reduced pressure to obtain a solid, and then recrystallized to obtain 5.8 g of a compound with the structure of formula IV. The yield was 71%, where X is Br in the formula IV, represented by IV-Br, its nu...
Embodiment 3
[0055] Embodiment 3: Mangiferin aglycon is prepared by the method of the present invention
[0056] Weigh 5.40g (0.02mol) of 2-iodo-4,5-dimethoxybenzoic acid and 3.40g (0.02mol) of 1,3,5-trimethoxybenzene into a 100ml three-necked reaction flask, stir 2.0 ml of phosphorus oxychloride and 20 ml of solvent 1,2-dichloroethane were added. Heat the mixture to 120°C, stir and react for 10 hours, let it cool, add 50ml of water and 30ml of 1,2-dichloroethane, stir well and let it stand, separate the organic layer, and wash the water layer with 50ml of 1,2-dichloroethane Extract with alkane again, combine the organic layers, wash with water and semi-saturated aqueous sodium bicarbonate solution once, dry over anhydrous sodium sulfate, filter, and evaporate the filtrate to obtain a solid under reduced pressure to remove the solvent, and then recrystallize to obtain compound 5.9 with the structure of formula IV g, the yield is 72%, wherein X is I in formula IV, expressed with IV-I, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com