Method for preparing high-purity esomeprazole
A technology for esomeprazole salt and benzimidazole, which is applied in the field of synthesis and purification of esomeprazole salt, can solve the problems of increasing the difficulty of operation, unsuitable for industrial production, and high reaction temperature, and achieves improved drug efficacy and safety, reduce the difficulty of operation, reduce the effect of impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
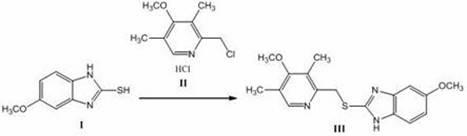
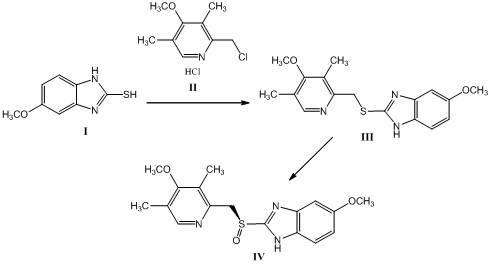
[0033] Embodiment 1 Esomeprazole ( )Synthesis
[0034]
[0035] Weighing type Add 180g (1mol) of the compound shown, add 540mL of methanol, and stir to obtain a suspension solution; weigh 100g (2.5mol) of NaOH and dissolve it in 100mL of water, add 200mL of methanol to obtain a mixed solution, slowly add to the methanol suspension of the above-mentioned imidazole In the solution, maintain the reaction temperature at about 50°C, and start to add slowly The methanol solution of the compound [Formula Compound 221g (1mol): Methanol 700mL]. After the dropwise addition, keep the temperature for 0.5-1h, monitor by TLC (petroleum ether: ethyl acetate = 1:3 as the developing agent), the reaction is complete, and the reaction is ended. After extraction, suction filtration and vacuum drying, 243g of formula The indicated compound has an HPLC purity of 99.76%, m.p.: 119-120°C.
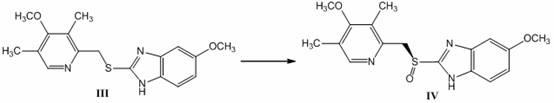
[0036] Under the protection of nitrogen, take the formula obtained in the previous step 229g...
Embodiment 2
[0038] The preparation of embodiment 2 esomeprazole sodium
[0039] Dissolve 35.0g of esomeprazole in 40mL of methanol, stir until dissolved and clear, and dissolve 5.0g of sodium hydroxide in 5.5mL of water, cool down to below room temperature, then dilute with 5.5mL of methanol, and add dropwise to the reaction solution , stirred at 45°C for 0.5h, then heated to 55°C for 1h. Concentrate, repeatedly add water with anhydrous isopropanol (25 mL×2), concentrate until viscous, add 7 mL ether and stir evenly, after standing still, a large amount of solid precipitates, pour 20 mL ether to dilute, and centrifuge to obtain 36.5 g Crude esomeprazole sodium.
[0040] Dissolve the crude esomeprazole sodium prepared in the previous step in 40mL of absolute ethanol in batches, control the system at about 35°C, and basically dissolve (slightly mixed); filter the above solution with a Buchner funnel containing activated carbon cake to obtain clarification yellow liquid. When the oil in...
Embodiment 3
[0047] The preparation of embodiment 3 esomeprazole sodium
[0048] Dissolve 35.0g of esomeprazole in 40mL of methanol, stir until dissolved and clear, and dissolve 5.0g of sodium hydroxide in 5.5mL of water, cool down to below room temperature, then dilute with 5.5mL of methanol, and add dropwise to the reaction solution , stirred at 45°C for 0.5h, then heated to 50°C for 1h. Concentrate, repeatedly add water with anhydrous isopropanol (25 mL×2), concentrate to a viscous substance, add 7 mL petroleum ether and stir evenly, after standing still, a large amount of solid precipitates, pour 20 mL petroleum ether to dilute, and centrifuge to obtain 35.7g crude esomeprazole sodium.
[0049] Dissolve the crude esomeprazole sodium prepared in the previous step in batches in 40mL of methanol, control the system at about 35°C, and basically dissolve (slightly mixed); filter the above solution with a Buchner funnel containing activated carbon cake to obtain a clear yellow liquid . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com