Bosentan sustained-release matrix tablet and preparation method thereof
A technology of bosentan and sustained-release tablets, applied in the field of medicine, can solve problems such as side effects, short multiple doses, unsolved half-life of drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
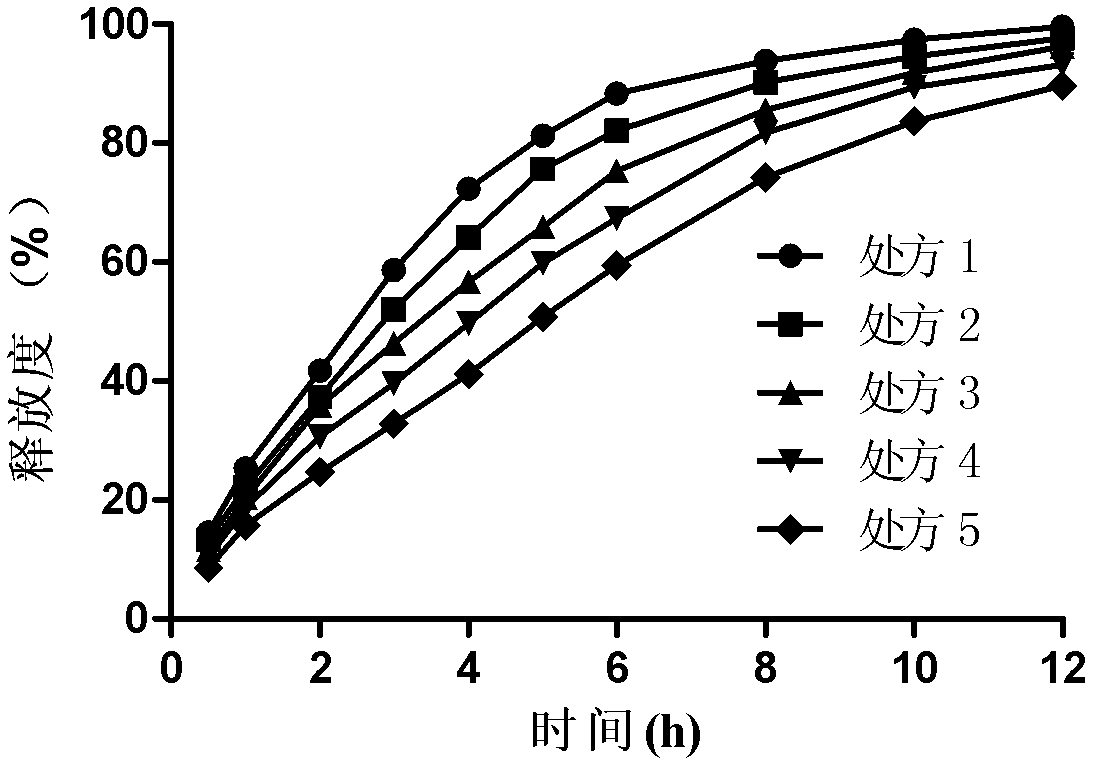
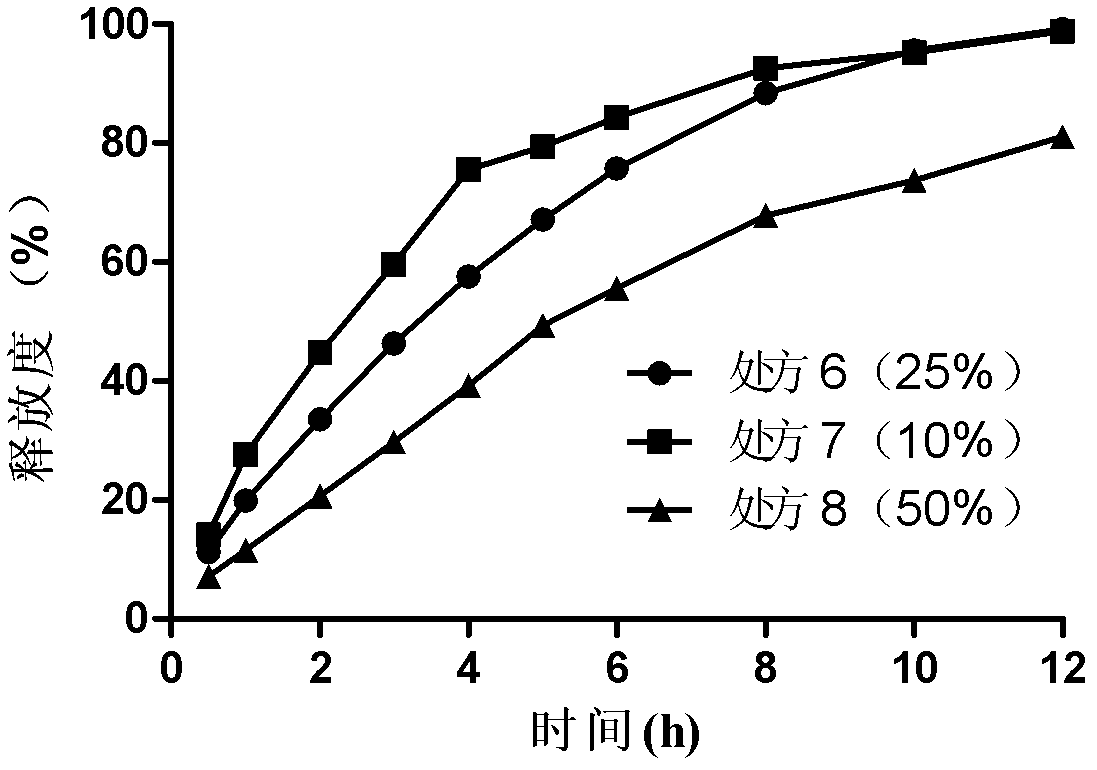
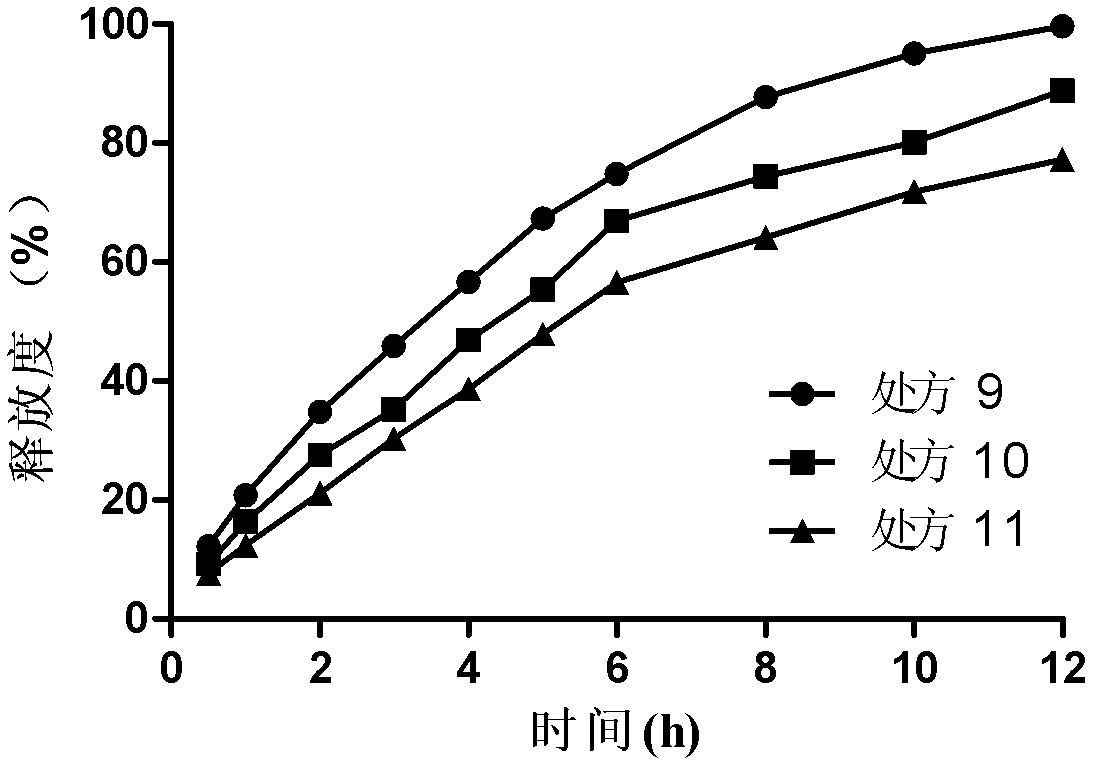
[0035] Example 1 Bosentan Gel Matrix Sustained Release Tablets (Specification: 250mg / tablet, 1000 tablets):
[0036]
[0037]
[0038] Tablet weight about 600mg
[0039] Dry direct compression: Weigh 250g of bosentan and the prescribed amount of excipients (HPMC K4M, K15M, sodium alginate, lactose, microcrystalline cellulose and sodium bicarbonate) and grind them through a 100-mesh sieve and mix, then add stearic acid Magnesium is directly compressed after mixing.
Embodiment 2
[0040] Example 2 Bosentan Gel Matrix Sustained Release Tablets (Specification: 250mg / tablet, 1000 tablets):
[0041]
[0042] Tablet weight about 600mg
[0043] Wet granulation and tabletting: Weigh 250g of bosentan, crush it through a 100-mesh sieve, mix it with the prescribed amount of excipients (HPMC K4M, K15M, sodium alginate, lactose, microcrystalline cellulose and sodium bicarbonate) and add an appropriate amount of moistening Wet agent ethanol aqueous solution (70%) is made into soft materials, extruded through a 20-mesh sieve to obtain wet granules, dried at 45-50°C for granulation, then added with lubricant magnesium stearate, mixed evenly, and then compressed into tablets.
Embodiment 3
[0044] Example 3 Bosentan Gel Matrix Sustained Release Tablets (Specification: 125mg / tablet, 1000 tablets):
[0045]
[0046] Tablet weight about 400mg
[0047] Preparation method is the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com