High-selectivity catalyst for naphthalene hydrogenation reaction for preparing tetrahydronaphthalene and preparation method thereof
A high-selectivity, catalyst technology, applied in the direction of molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems that precious metals cannot meet industrial production, achieve broad industrial application prospects, shorten pyrolysis process, avoid Paradoxical effect of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
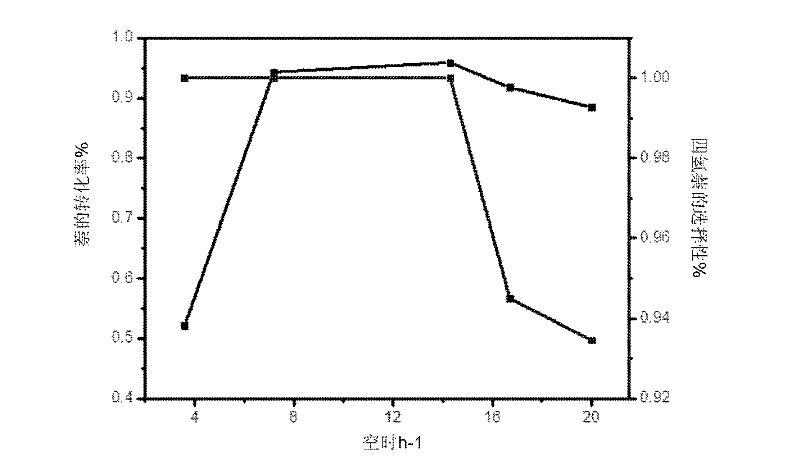
Examples
Embodiment 1
[0020] The preparation of embodiment 1 activated carbon supported molybdenum carbide catalytic material
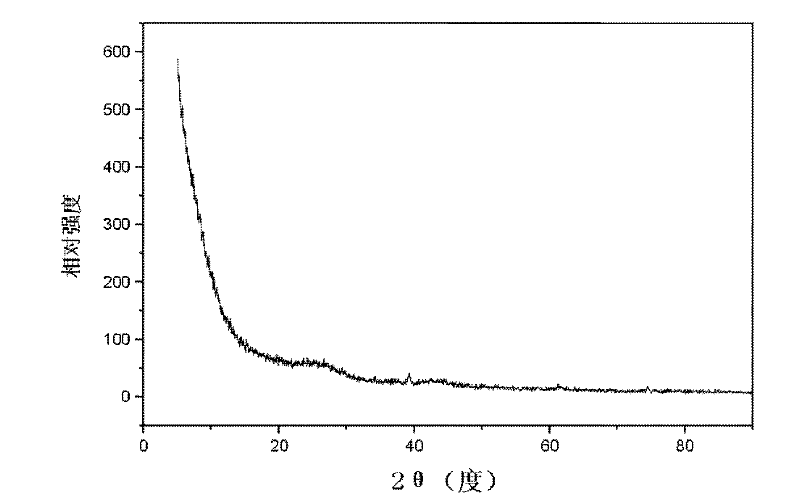
[0021] A certain amount of molybdenum-hexamethylenetetramine complex was added into 1 milliliter of ammonia water with a concentration of 15%. After fully dissolving, 0.1 g of activated carbon was weighed and added to the solution, and the solvent was naturally evaporated at room temperature. °C for 3 hours to obtain the precursor of molybdenum carbide. Molybdenum carbide precursors with different molybdenum loadings can be prepared by changing the amount of molybdenum-hexamethylenetetramine complex added. Place the precursor in a microwave reactor, blow it with argon for 2 hours, turn on the microwave at 800W and heat it for 30min under an argon atmosphere. After the sample is cooled, it is identified as Mo by X-ray powder diffraction. 2 c.
Embodiment 2
[0022] The preparation of the molybdenum carbide catalytic material of embodiment 2SBA-15 loading nickel modification
[0023] A certain amount of molybdenum-hexamethylenetetramine complex is added to 1 ml of 15% ammonia water, and 1 ml of 15% ammonia water is added. After fully dissolving, add 0.1 g of activated carbon into the solution, evaporate the solvent naturally at room temperature, and dry in a vacuum oven at 80°C for 3 hours to obtain a nickel-modified molybdenum carbide precursor. Nickel-modified molybdenum carbide precursors with different loadings of molybdenum and nickel can be prepared by changing the addition amount of molybdenum-hexamethylenetetramine complex and nickel nitrate hexahydrate. Since the molybdenum carbide precursor modified by SBA-15 loaded with nickel does not absorb microwaves and cannot heat itself under the action of microwaves, 0.05g of zirconia is mixed with the precursor and placed in a microwave reactor (zirconia is used to absorb microwa...
Embodiment 3 2
[0024] The preparation of the molybdenum carbide catalytic material of embodiment 3 silica loaded cobalt modification
[0025] A certain amount of molybdenum-hexamethylenetetramine complex is added to 1 ml of 15% ammonia water, and 1 ml of 15% ammonia water is added. After being fully dissolved, 0.1 g of silicon dioxide was weighed and added to the solution, the solvent was naturally evaporated at room temperature, and dried in a vacuum oven at 80°C for 3 hours to obtain a cobalt-modified molybdenum carbide precursor. Cobalt-modified molybdenum carbide precursors with different loadings of molybdenum and cobalt can be prepared by changing the addition amount of molybdenum-hexamethylenetetramine complex and cobalt chloride hexahydrate. Since the silica-supported cobalt-modified molybdenum carbide precursor does not absorb microwaves and cannot heat itself under the action of microwaves, 0.05g of zirconia is mixed with the precursor and placed in a microwave reactor (zirconia is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com