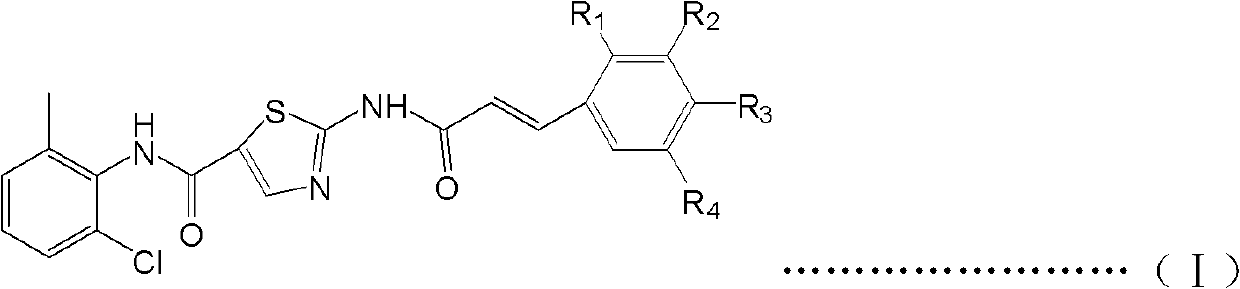
N-(2-chloro-6-methyl phenyl)-2-(phenyl acrylamide)thiazole-5-formamide derivative, and preparation method and use thereof
A technology of phenylacrylamide and methylphenyl, which is applied in the field of its preparation, N--2-thiazole-5-carboxamide derivatives, can solve the problems of white blood cell concentration surge and uncontrollable growth of white blood cells, and achieve obvious inhibitory effect , novel structure and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1N-(2-chloro-6-methylphenyl)-2-(3,4-dimethylphenylacrylamide)thiazole-5-carboxamide
[0034]
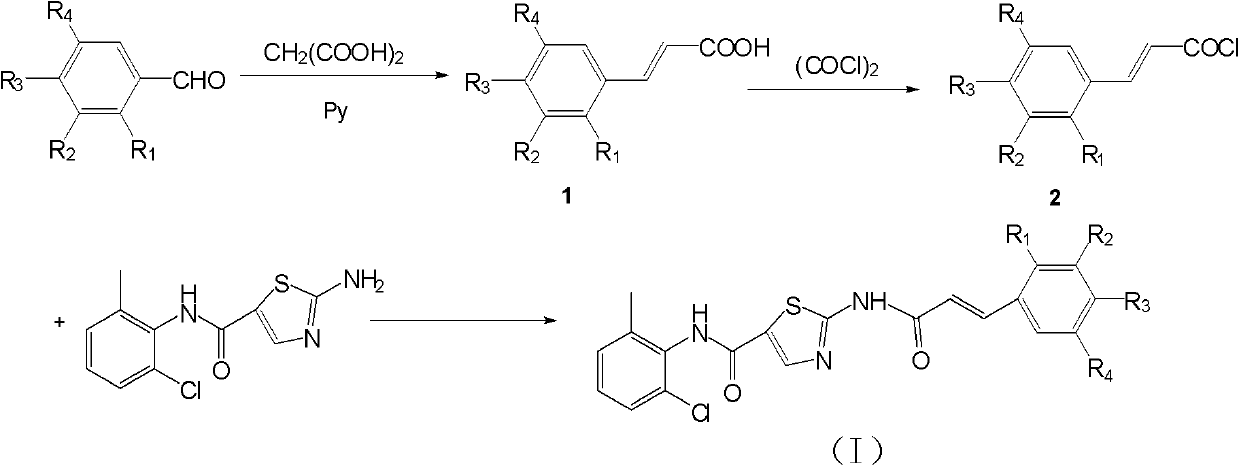
[0035] synthetic route:
[0036]
[0037] 1. Preparation of intermediate 1-3,4-dimethylphenylacrylic acid
[0038] Add 1.34g (10.0mmol) of 3,4-dimethylbenzaldehyde, 2.08g (20.0mmol) of malonic acid, 8mL of pyridine and 0.5mL of piperidine respectively in a 100mL three-necked flask, and heat the oil bath to reflux temperature for reaction. After 6 hours, thin-layer chromatography (TLC) was used to detect that 3,4-dimethylbenzaldehyde had reacted completely (developing solvent is the solvent obtained by mixing ethyl acetate, sherwood oil and glacial acetic acid in a volume ratio of 1:6:1), Stop the reaction, add 8 mL of toluene to the reaction system, then add K 2 CO 3solution, adjust the pH to 9-10, stir for 30 minutes, then separate the liquid to retain the water layer, adjust the pH to 3 with 1mol / L HCl, at this time, a large amount of white solid precipit...
Embodiment 2
[0044] Embodiment 2N-(2-chloro-6-methylphenyl)-2-(3,5-dimethoxyphenylacrylamide)thiazole-5-carboxamide
[0045]
[0046] The operating steps are the same as in Example 1, except that in step 1, 3,5-dimethoxybenzaldehyde is used instead of 3,4-dimethylbenzaldehyde. The target product is a white solid with a melting point of 309-310°C and a yield of 92.1%.
[0047] 1 H NMR (400MHz, DMSO-d 6 )δ: 12.64 (s, 1H), 10.07 (s, 1H), 8.32 (s, 1H), 7.68-7.75 (d, 1H, J=15.8Hz), 7.24-7.43 (m, 3H), 6.82-6.96 (m, 3H), 6.6 (d, 1H, J=4.4Hz), 3.8 (s, 6H), 2.27 (s, 3H).
Embodiment 3
[0048] Example 3N-(2-chloro-6-methylphenyl)-2-(3-trifluoromethylphenylacrylamide)thiazole-5-carboxamide
[0049]
[0050] The operating steps are the same as in Example 1, except that in step 1, 3-trifluoromethylbenzaldehyde is used instead of 3,4-dimethylbenzaldehyde. The target product is a white solid with a melting point of 284-286°C and a yield of 92.5%.
[0051] 1 H NMR (400MHz, DMSO-d 6 )δ: 12.60(s, 1H), 10.01(s, 1H), 8.31(s, 1H), 7.78-8.05(m, 4H), 7.68-7.75(d, 1H), 7.2-7.45(m, 3H) , 7.03-7.08 (d, 1H, J=15.9Hz), 2.27 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com