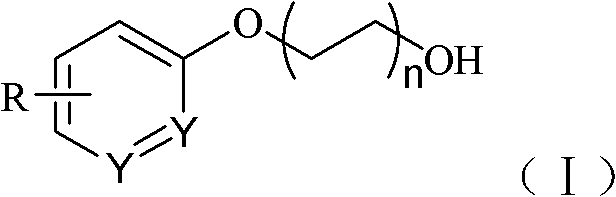
Synthetic method of diol phenylate compounds
A synthetic method, a technology of diol phenyl ether, applied in the preparation of organic compounds, chemical instruments and methods, the formation/introduction of ether groups/acetal groups/ketal groups, etc., can solve the problem of corrosiveness and low yield of products , dangerous epoxy compounds and other issues, to achieve the effect of non-corrosive preparation and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of glycol phenyl ether compounds shown in embodiment 1, formula (II)
[0021]
[0022] Put 0.102g of iodobenzene (0.5mmol), 0.0095g of cuprous iodide (0.05mmol), and 0.276g of potassium carbonate (2mmol) into a 50ml reaction vessel dried in an oven, and inject 0.248g of ethylene di Alcohol (4mmol), 2ml toluene, in this reaction system, the mol ratio of iodobenzene and glycol is 1: 8, and the mol ratio of iodobenzene and potassium carbonate is 1: 4, and cuprous iodide accounts for the molar percentage of iodobenzene The content was 10%, reacted for 24 hours, extracted with ethyl acetate and concentrated with a rotary evaporator and passed through the column (ethyl acetate:petroleum ether=4:1, v / v) to obtain 28.4mg of product with a yield of 41.2%IR (film )vmax3391, 3063, 3040, 2932, 2876, 1599, 1495, 1455, 1246; 1 H NMR (500MHz, CDCl 3 )δ2.05(s, 1H3.97(t, J=4.0Hz, 2H), 4.09(t, J=4.0Hz, 2H), 6.92-6.99(m, 3H), 7.29(d, J=12Hz, 2H); 13 C NMR (500MHz, CDCl 3...
Embodiment 2
[0023] Synthesis of glycol phenyl ether compounds shown in embodiment 2, formula (II)
[0024] With 0.102g iodobenzene (0.5mmol), inject 0.031g ethylene glycol (0.5mmol), 2ml toluene, in this reaction system, the molar ratio of iodobenzene and diol is 1: 1, other conditions are as embodiment 1, obtain 6.9mg product, yield 10%
Embodiment 3
[0025] Synthesis of diol phenyl ether compounds shown in embodiment 3, formula (II)
[0026] With 0.102g iodobenzene (0.5mmol), inject 0.124 ethylene glycol (2mmol), 2ml toluene, in this reaction system, the molar ratio of iodobenzene and diol is 1: 4, other conditions are as embodiment 1, obtain 18.63mg product, yield 27%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com