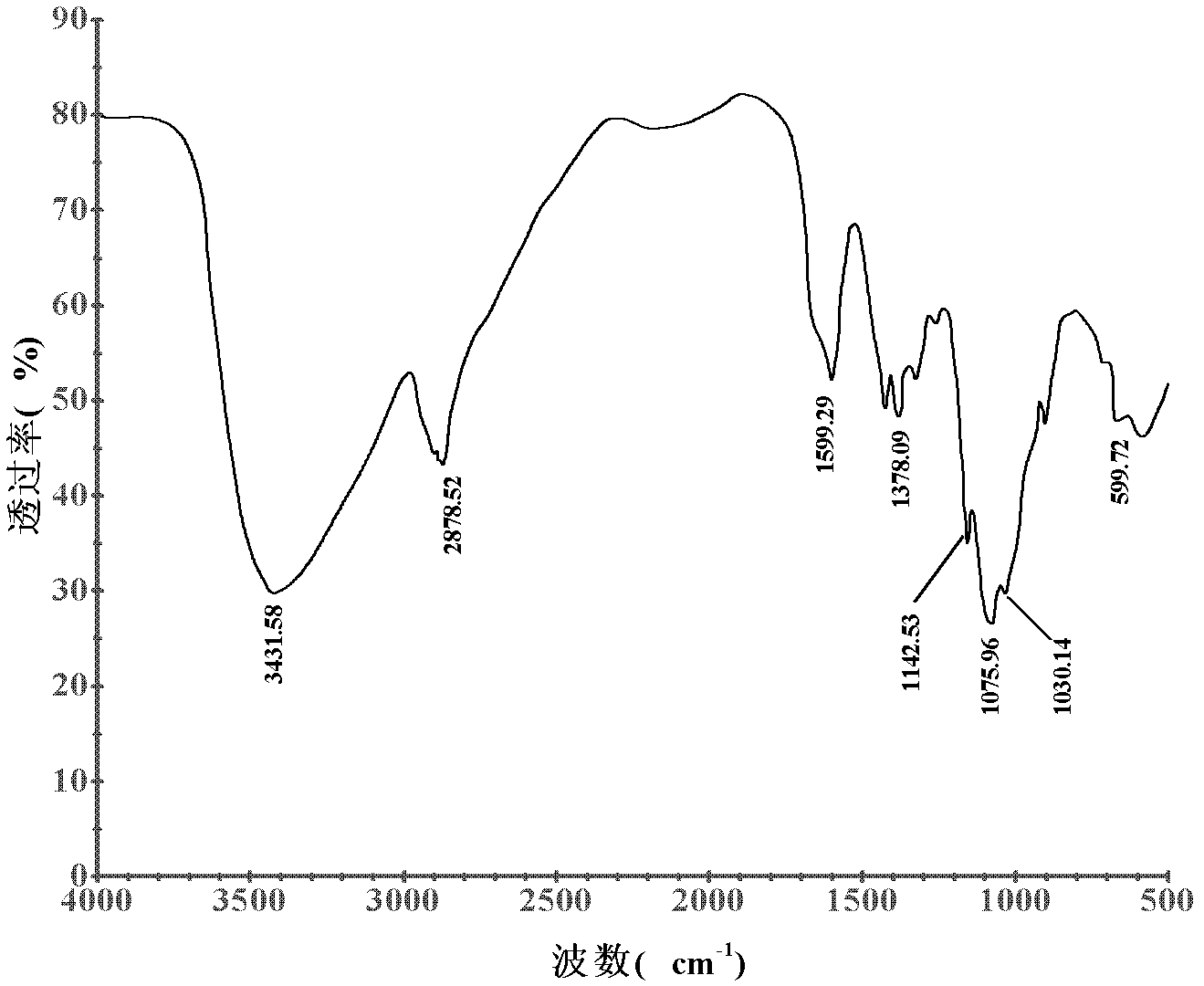
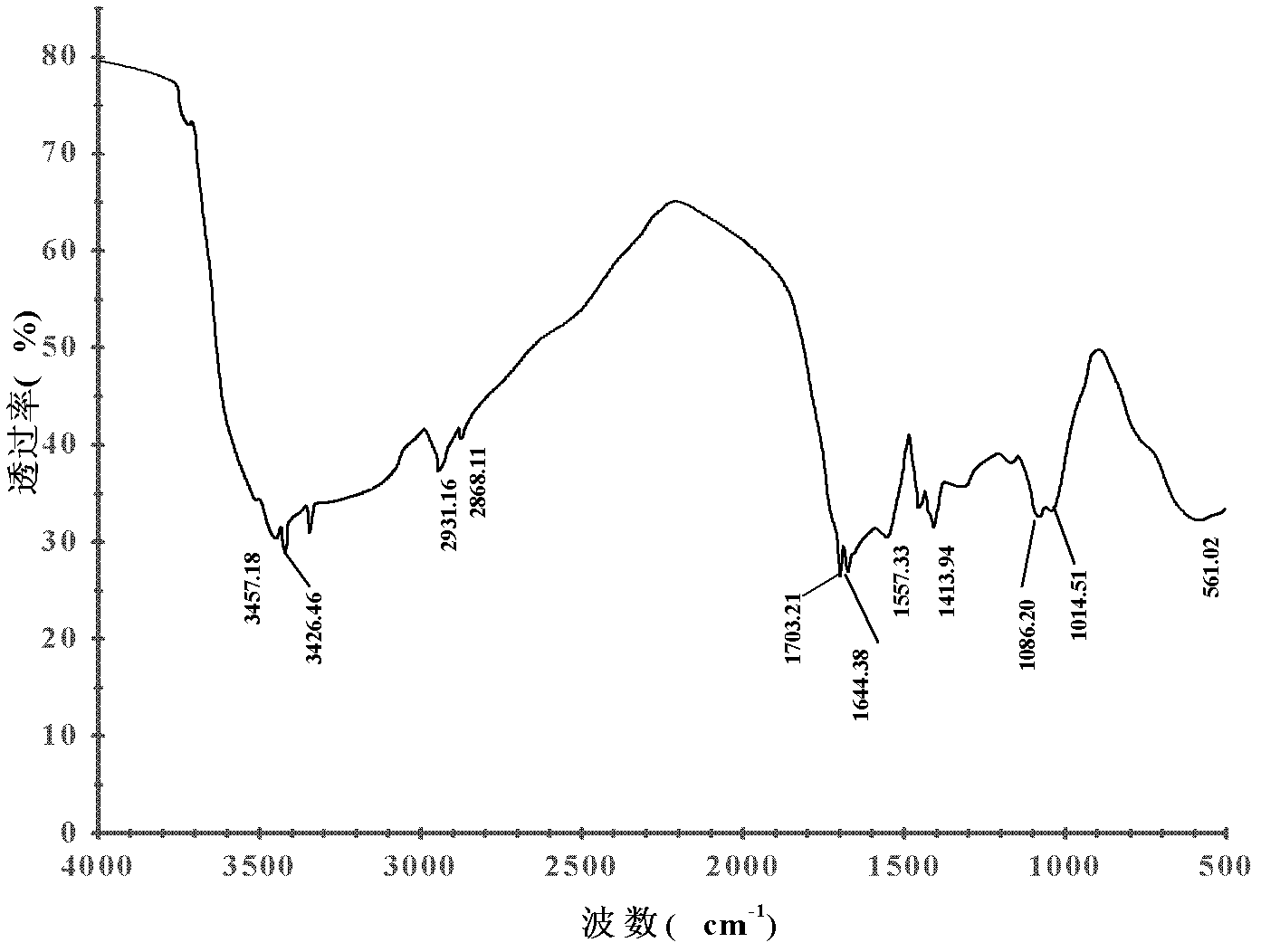
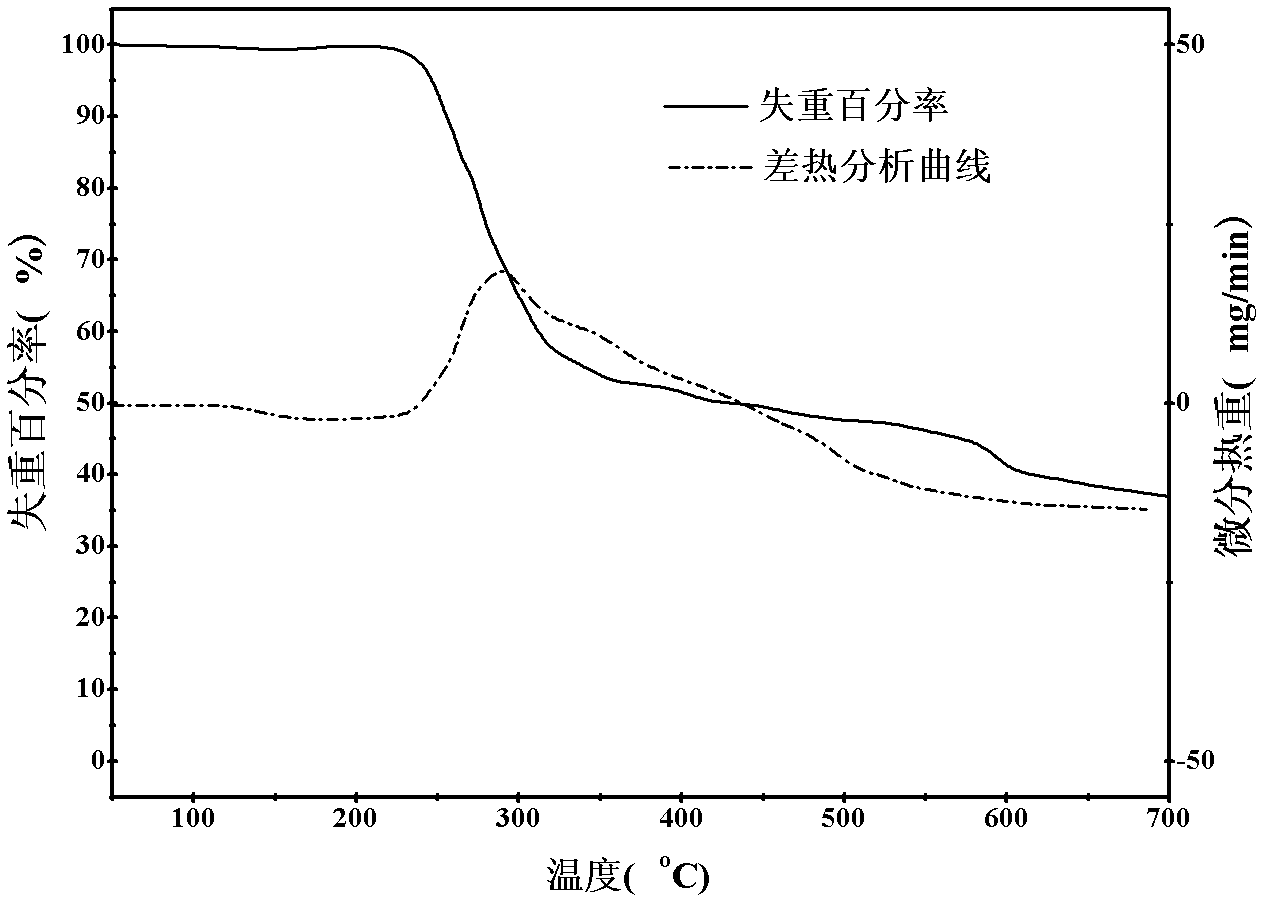
Chitosan derivative and preparation method thereof
A chitosan derivative, chitosan technology, applied in the field of polymer chemistry, can solve the problems of small molecular weight, high use cost, poor bridging ability, etc., and achieve the effect of good adsorption performance and improved removal ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take 5 g of dried chitosan and put it into a four-neck bottle, add 400ml of 2% wt acetic acid, and heat to 80°C on a water bath until all the chitosan is dissolved in acetic acid. Cool the chitosan acetic acid solution to 45° C., add 0.65 g of potassium peroxodisulfate and 0.25 g of sodium bisulfite under nitrogen protection, and stir mechanically for 30 minutes. Add 21 g of acrylamide into the four-necked bottle, and drop 6 g of acrylic acid into it through a constant pressure dropping funnel within 90 minutes, then continue to react at 45 degrees Celsius for 1 hour. Adjust the pH of the reaction solution to about 7 with 0.5 mol / L sodium hydroxide to obtain a white flocculent precipitate, which is suction-filtered at room temperature, washed with acetone and then suction-filtered to obtain a crude product. The crude product was dried and extracted with N,N-dimethylformamide Soxhlet for 12 hours, then filtered with suction, and dried in vacuum to obtain chitosan derivat...
Embodiment 2
[0036] Take 5 g of dried chitosan and put it into a four-neck bottle, add 400ml of 2% wt acetic acid, and heat to 80°C on a water bath until all the chitosan is dissolved in acetic acid. Cool the chitosan acetic acid solution to 30° C., add 0.65 g of potassium peroxodisulfate and 0.07 g of sodium bisulfite under nitrogen protection, and stir mechanically for 30 minutes. 22.5 g of acrylamide was added into the four-neck flask, and at the same time, 7.5 g of acrylic acid was dropped into it through a constant pressure dropping funnel within 2 hours, and the reaction was continued at 55° C. for 1 hour. The pH value of the reaction solution was adjusted to about 7 with sodium hydroxide to obtain a white flocculent precipitate, which was suction filtered at room temperature, washed with acetone and then suction filtered to obtain a crude product. The crude product was extracted with N,N-dimethylformamide Soxhlet for 12 hours, then suction-filtered, and vacuum-dried to obtain the pr...
Embodiment 3
[0038] Take 5 g of dried chitosan and put it into a four-neck bottle, add 400ml of 2% wt acetic acid, and heat to 80°C on a water bath until all the chitosan is dissolved in acetic acid. Cool the chitosan acetic acid solution to 45° C., add 0.65 g of potassium peroxodisulfate and 0.12 g of sodium bisulfite under nitrogen protection, and stir mechanically for 30 minutes. 11.6 g of acrylamide was added into the four-neck flask, and at the same time, 4.5 g of acrylic acid was dropped into it through a constant pressure dropping funnel within 2.5 hours, and the reaction was continued at 40 degrees Celsius for 40 minutes. Adjust the pH of the reaction solution to about 7 with 0.5 mol / L sodium hydroxide to obtain a white flocculent precipitate, which is suction-filtered at room temperature, washed with acetone and then suction-filtered to obtain a crude product. The crude product was dried and extracted with N,N-dimethylformamide Soxhlet for 10 hours, then suction-filtered, and vacu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com