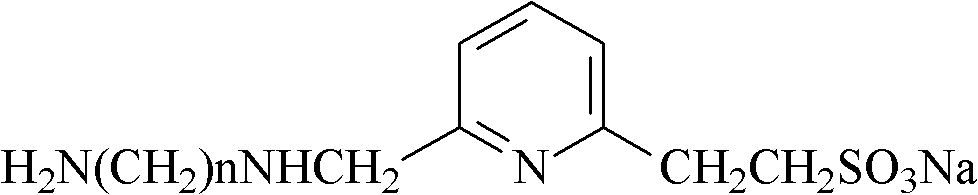
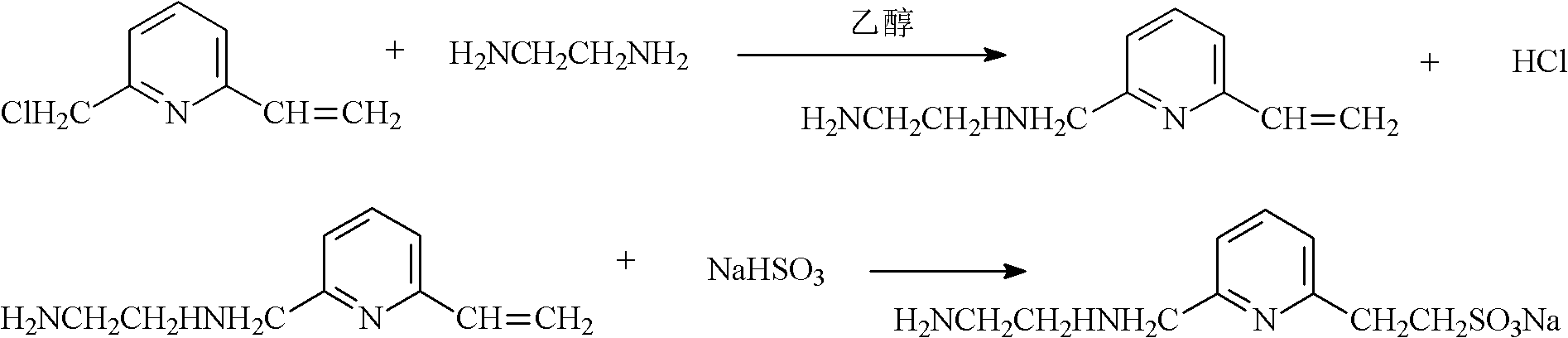
Preparation method of pyridine sulfonic acid type water-based polyurethane emulsion
A water-based polyurethane, pyridine sulfonic acid technology, applied in the direction of polyurea/polyurethane adhesives, adhesive types, adhesives, etc., can solve the problem of water-based polyurethane crystallization properties, initial viscosity, physical shrinkage resistance, and yellowing resistance. and other problems, to achieve the effect of good resistance to physical shrinkage, improve crystallization performance, and improve initial bond strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The preparation method of this pyridine sulfonic acid type aqueous polyurethane emulsion comprises the following steps:
[0015] (1) Carry out vacuum dehydration treatment to polyester polyol, then carry out prepolymerization reaction with organic diisocyanate, dimethylol propionic acid (DMPA) in the presence of catalyst, the reaction time is 2~5 hours, preferably 4h; Reaction The temperature is 70-100°C, preferably 75-85°C;
[0016] (2) Cool the prepolymer obtained in the previous step to below 50°C, then dissolve it with acetone, then add a salt-forming reagent to neutralize, and the neutralization time is 10 to 30 minutes;
[0017] (3) Add pyridine sulfonic acid diamine hydrophilic chain extender to the material obtained in the previous step to carry out chain extension reaction to obtain a polymer with an NCO group in the end group. The reaction temperature is 10-60°C, and the reaction time is 0.5- 1h. The described pyridinesulfonic acid diamine hydrophilic chain ...
Embodiment 1
[0036] In the reactor with agitator, thermometer and cooler, add 200g of PBA (the number average molecular weight is 2000), melt at 100~120°C, vacuum dehydrate for 1h, and start to cool down when the moisture content is less than 0.1% by sampling; When the temperature dropped to 80°C, 13.3g IPDI, 19.5g HDI, 11g DMPA and 0.05g DBTDL catalyst were added for prepolymerization reaction, and the prepolymer was obtained after stirring for about 4 hours. Cool the above prepolymer to 50°C, add 170g of acetone to dissolve the prepolymer, then add 0.7g of triethylamine for neutralization, and the neutralization time is 15min; add 4.2g of 2-[6-(2-aminoethylamino) Pyridyl]sodium ethyl sulfonate was subjected to a chain extension reaction, and the reaction time was 0.5h to obtain a polymer with an NCO group in the end group; 210g of deionized water was added to the above polymer, stirred at a high speed to disperse into an emulsion, and then added 1.5g of ethylenediamine was used for secon...
Embodiment 2
[0039]In the reactor with stirrer, thermometer, cooler, add 200g PBA (the number average molecular weight is 2000), melt at 100~120 ℃, vacuum dehydration 1h, begin to cool down when the water content of sampling measurement is less than 0.1%; When the temperature dropped to 80°C, 38.1g IPDI, 3.1g DMPA and 0.05g DBTDL catalyst were added for prepolymerization reaction, and the prepolymer was obtained after stirring for about 4 hours. Cool the above prepolymer to 50°C, add 210g of acetone to dissolve the prepolymer, then add 2.1g of triethylamine for neutralization, and the neutralization time is 15min; add 3.5g of 2-[6-(2-aminopropylamino) Pyridyl]sodium ethyl sulfonate was subjected to a chain extension reaction, and the reaction time was 0.5h to obtain a polymer with an NCO group in the end group; 225g of deionized water was added to the above polymer, stirred at a high speed to disperse into an emulsion, and then added 1.5g of ethylenediamine was used for secondary chain ext...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com