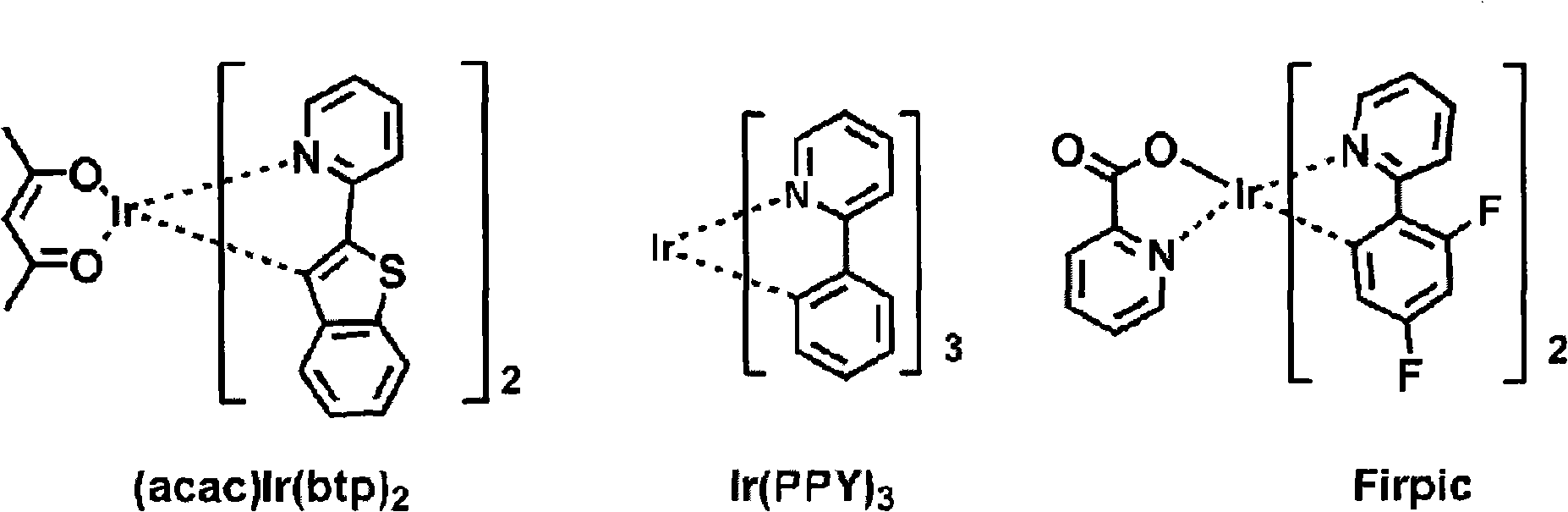
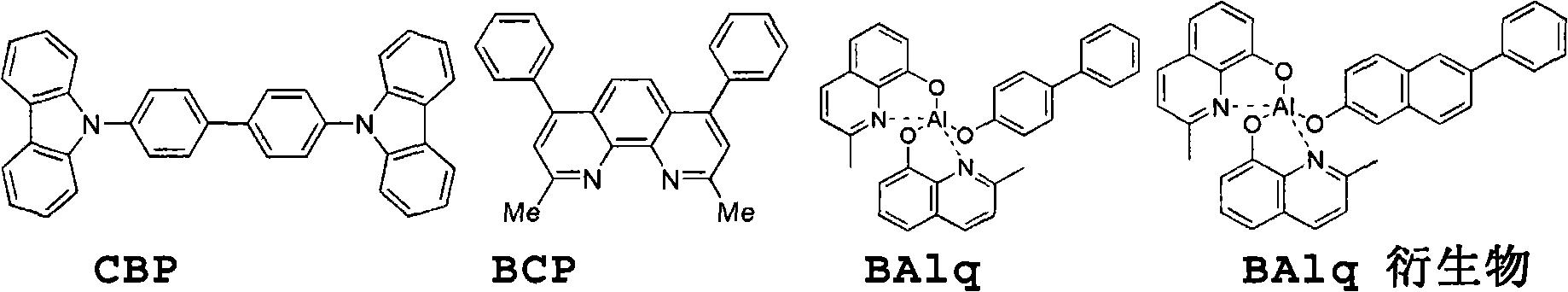
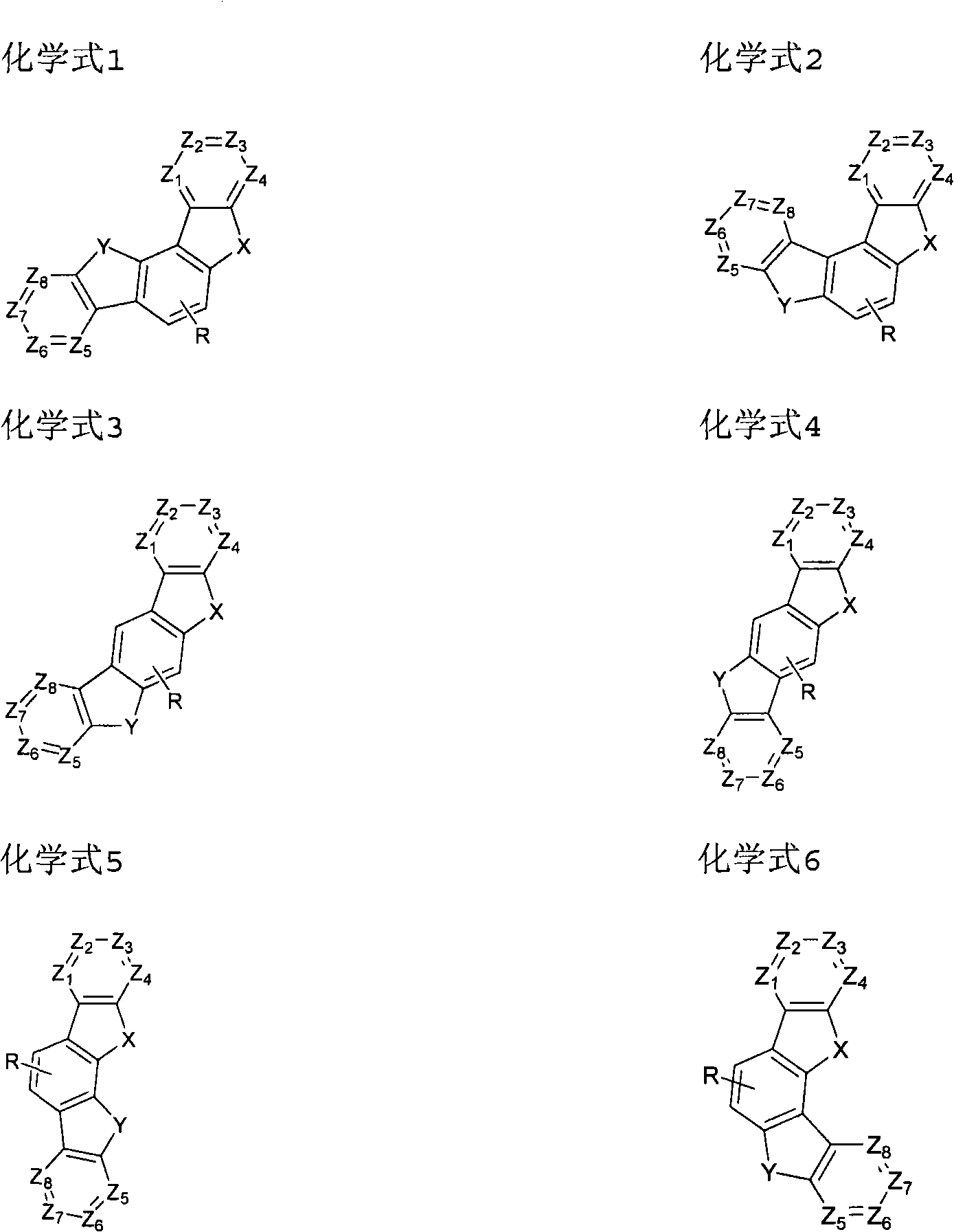
Novel organic electroluminescent compounds and organic electroluminescent device using the same
An electroluminescence device and luminescence technology, which are applied in electroluminescence light sources, organic chemistry, compounds of group 4/14 elements of the periodic table, etc., can solve the problem of no obvious advantage in power efficiency, OLED equipment does not have a working life, High driving voltage and other issues, to achieve good luminous efficiency, good working life, and excellent life properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0052] [Preparation Example 1] Preparation of Compound A
[0053]
[0054] Preparation of Compound A-1
[0055] Mix 1-bromonitrobenzene (16g, 74.25mmol), 9,9-dimethyl-9H-fluoren-2-ylboronic acid (23g, 96.60mmol), Pd(PPh 3 ) 4 (4.2g, 3.63mmol), 2M K 2 CO 3 Aqueous solution (111 mL), EtOH (100 mL) and toluene (200 mL), and heated to reflux at 120° C. for 3 hours. After the reaction was completed, the mixture was washed with distilled water. Extraction with EA and MgSO 4 After drying the organic layer, the solvent was removed using a rotary evaporator. The residue was purified by column chromatography to obtain compound (A-1) (22 g, 95%).
[0056] Preparation of Compound A-2
[0057] Compound A-1 (24 g, 76.10 mmol), triethyl phosphite (200 mL) and 1,2-dichlorobenzene (200 mL) were mixed, heated to 180° C., and stirred for 12 hours. When the reaction was completed, unreacted triethyl phosphite and 1,2-dichlorobenzene were removed using a distillation apparatus, and the...
preparation example 2
[0066] [Preparation Example 2] Preparation of Compound B
[0067]
[0068] Preparation of Compound B-2
[0069] Compound B-1 (50.0 g, 179 mmol) was dissolved in DMF (200 mL), and copper powder (27.0 g, 424 mmol) was added thereto. The mixture was stirred at 125°C for 3 hours. The reaction mixture was cooled at room temperature, filtered and the precipitate was removed and dried. Washing with MeOH (500 mL) gave compound B-2 (27.1 g, 88%).
[0070] Preparation of Compound B-3
[0071]Compound B-2 (15 g, 37.3 mmol) was dissolved in ethanol (200 mL), and 32% (w / w) HCl aqueous solution (120 mL) was added thereto. Tin powder (17.6 g, 147 mmol) was added in portions within 10 minutes at room temperature, and stirred at 100° C. for 2 hours. After cooling at room temperature, the reaction mixture was added to ice water and made basic using 20% (w / w) aqueous NaOH (150 mL). Extracted with diethyl ether, washed with brine (bryn) and dried. Recrystallization from ethanol gave C...
preparation example 3
[0089] [Preparation Example 3] Preparation of Compound C
[0090]
[0091] Preparation of Compound C-1
[0092] Compound C-1 (1.7 g, 50%) was prepared in the same manner as Compound B-5 in Preparation 2, using Compound B-4 as a starting material, except that dichlorodiphenylsilane was used instead of dichlorodimethylsilane.
[0093] Preparation of Compound C
[0094] Compound C (347 mg, 55%) was prepared in the same manner as Compounds B-6, B-8, B-9, B-10 and B in Preparation 2 using Compound C-1 as a starting material.
[0095] Prepare organic electroluminescence compound TA, TB and TC according to the method for preparation example 1-3, table 1-4 has listed 1 H NMR and MS / FAB, which are substituted versions of the prepared organic electroluminescent compounds.
[0096] Table 1
[0097]
[0098]
[0099]
[0100]
[0101]
[0102] Table 2
[0103]
[0104]
[0105]
[0106]
[0107] table 3
[0108]
[0109]
[0110]
[0111] Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com