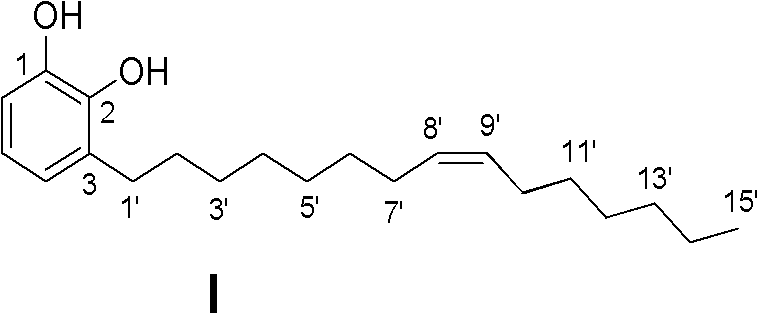
Preparation method for urushiol compound
A compound, urushiol technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., to achieve the effect of shortening time, avoiding thermal instability, and fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take 50.2 grams of raw lacquer, dissolve it with ethyl acetate, mix it with silica gel (100-200 mesh), evaporate the solvent, and set aside.
[0027] Get 300 grams of silica gel, use sherwood oil-ethyl acetate (30:1) wet packing column, column diameter 5cm, column length 35cm; The sample that mixes is added to the top of column, with petroleum ether-ethyl acetate (30:1) : 1) for elution, the flow rate is 1BV / h, the front 1000ml eluent is discarded, then every 100ml is collected in one portion, the fractions are detected by HPLC, 22-36 are combined, and the solvent is reclaimed under reduced pressure to obtain 4.1g of the sample.
[0028] Take by weighing 50g C-18 reverse-phase material, wet packing column, column diameter is 2.1cm, column length 21cm, takes by weighing the sample 1.25g that gains by silica gel column chromatography, dissolves with methanol, loads sample, with methanol-water ( 80:20) elution, the flow rate is 2ml / min, 20ml collects a fraction, collects 2...
Embodiment 2
[0031] Take 50.8 grams of raw lacquer, dissolve it with chloroform, mix it with silica gel (100-200 mesh), evaporate the solvent, and set aside.
[0032]Get 200 grams of silica gel, and use petroleum ether-ethyl acetate (20:1) wet packing column, column diameter 5cm, column length 22cm; : 1) for elution, the flow rate is 1.5BV / h, the front 900ml eluent is discarded, then every 100ml is collected in one portion, the fractions are detected by HPLC, 11-26 are combined, and the solvent is reclaimed under reduced pressure to obtain 4.2g of sample.
[0033] Weigh 80g of C-18 reverse-phase material, wet-pack the column with a column diameter of 2.1cm and a column length of about 33cm, weigh 1.31g of the sample obtained by silica gel column chromatography, dissolve it in methanol, load the sample, and use methanol-water (75:25) elution, flow rate is 1ml / min, 20ml collects a fraction, collects 260 fractions altogether, detects with HPLC, merges the 174th-258th fraction that contains ta...
Embodiment 3
[0036] Take 51.2 grams of raw lacquer, dissolve it with chloroform, mix it with silica gel (100-200 mesh), evaporate the solvent, and set aside.
[0037] Get about 400 grams of silica gel and wet-pack the column with petroleum ether-ethyl acetate (15:1), with a column diameter of 6 cm and a column length of 31 cm; add the mixed sample to the top of the column, and use petroleum ether-ethyl acetate ( 15:1) for elution, the flow rate is 2BV / h, the front 1500ml eluent is discarded, and then every 100ml is collected, and the fractions are detected by HPLC, 15-28 are combined, and the solvent is recovered under reduced pressure to obtain 4.25g of the sample.
[0038] Weigh about 100g of C-18 reversed-phase material and wet-pack the column with a diameter of 3cm and a column length of about 20cm. Weigh 1.28g of the sample obtained by silica gel column chromatography, dissolve it in methanol, load the sample, and use methanol-water (85:15) elution, flow rate is 1.5ml / min, about 20ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com