Method for production of tricyclohexylphosphine
A technology of tricyclohexylphosphine and hexylphosphine oxide, which is applied in the production field of tricyclohexylphosphine, can solve the problems of high price, difficult preparation of catalyst niobium metal complexes, and dangerous use, and achieves improved yield, good application prospects, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
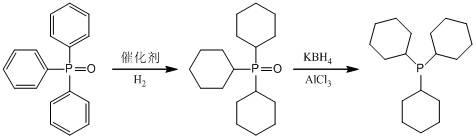
[0019] In a 1L autoclave, add 278 g (1 mol) triphenylphosphine oxide, 13.9 g catalyst Pd / Al 2 o 3 and 300 mL of tetrahydrofuran, nitrogen replacement 3 times and hydrogen replacement 3 times, catalytic hydrogenation at a reaction temperature of 180 °C and a hydrogenation pressure of 6.0 MPa, the reaction was stopped after the GC detection was complete, and the catalyst was removed by filtration to obtain a tricyclohexylphosphine oxide solution .
[0020] Under the protection of Ar gas, add 162g (3 mol) KBH into 2 L reactor 4 and 100 mL of diethyl ether, 133.5 g (1 mol) of anhydrous AlCl at a reaction temperature of 20 °C 3 The diethyl ether (300 mL) solution was dropped into the reactor, and then the tricyclohexylphosphine oxide solution obtained above was dropped into the KBH at this temperature 4 -AlCl 3 In the system, after dropping, the temperature was raised to reflux and stirred overnight. When there was no tricyclohexylphosphine oxide in the sampling analysis, it wa...
Embodiment 2
[0027] In a 1L autoclave, add 278 g (1 mol) of triphenylphosphine oxide, 2.78 g of catalyst Rh / C and 300 mL of dioxane, and replace 3 times with nitrogen and 3 times with hydrogen, at a reaction temperature of 190 °C, Catalytic hydrogenation was carried out at a hydrogenation pressure of 10.0 MPa. The reaction was stopped after GC detection was complete, and the catalyst was removed by filtration to obtain a tricyclohexylphosphine oxide solution.
[0028] Under the protection of Ar gas, add 194.4g (3.6 mol) KBH into 2 L reactor 4 and 200 mL of diethyl ether, 160 g (1.2 mol) of anhydrous AlCl at a temperature of 30 °C 3 The diethyl ether (400 mL) solution was dropped into the reactor, and then the tricyclohexylphosphine oxide solution obtained above was dropped into the KBH at this temperature 4 -AlCl 3 In the system, after dropping, the temperature was raised to reflux and stirred overnight. When there was no tricyclohexylphosphine oxide in the sampling analysis, it was hydr...
Embodiment 3
[0031] In a 1L autoclave, add 278 g (1 mol) of triphenylphosphine oxide, 7.8 g of catalyst Ru / C and 300 mL of cyclohexane. After three times of nitrogen replacement and three times of hydrogen replacement, at a reaction temperature of 200 °C, add Catalyzed hydrogenation under a hydrogen pressure of 8.0 MPa, stopped after the reaction was complete as detected by GC, and filtered to remove the catalyst to obtain tricyclohexylphosphine oxide solution.
[0032] Under the protection of Ar gas, add 243 g (4.5 mol) KBH into the 2 L reactor 4 and 300 mL of diethyl ether, 200 g (1.5 mol) of anhydrous AlCl 3 The diethyl ether (600 mL) solution was dropped into the reactor, and then the tricyclohexylphosphine oxide solution obtained above was dropped into the KBH at this temperature 4 -AlCl 3 In the system, after dropping, the temperature was raised to reflux and stirred overnight. When there was no tricyclohexylphosphine oxide in the sampling analysis, it was hydrolyzed and the organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com