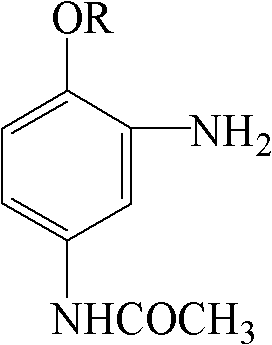Method for producing 2-amino-4-acetamido benzene alkyl ether by reduction with sodium bisulfide
A technology of acetaminophenyl alkyl ether and sodium hydrosulfide, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem that the quality cannot meet the production requirements, the control of the end point of acylation is difficult, and the process is complicated and other problems, to achieve the effect of simple equipment and operation, low cost and less waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 52.5 parts of wet 2-nitro-4-acetamidoanisole (dried) to 260 parts of water, add 6.3 parts of glacial acetic acid, and adjust the pH to 5 with liquid caustic; Add 21 parts of sodium hydrosulfide solution dropwise at a constant speed at 90-95°C (100%, solution concentration 25%); after the addition is complete, keep the temperature at 90-95°C for 1 hour, slowly cool down to below 30°C to precipitate crystals, filter, Wash the crystals with cold water until the pH of the filtrate = 7, and obtain 40.8 parts of 2-amino-4-acetamidoanisole (dried), the dry product-NH2 value is 98.70%, the HPLC purity is 99.44%, the initial melting point is 116.0 ° C, and the yield is 90.7% .
Embodiment 2
[0022] Add 56.0 parts of wet 2-nitro-4-acetamidophenethyl ether (dried) to 200 parts of water, add 2.8 parts of glacial acetic acid, and adjust the pH to 6 with soda ash; Add 28 parts of sodium hydrosulfide solution dropwise at a constant speed at 100°C (100% reduction, solution concentration 12.5%). After the dropwise addition, keep the reaction at 95-100°C for 1 hour. Slowly lower the temperature to below 30°C to precipitate crystals, filter, wash the crystals with cold water until the filtrate pH = 7, and obtain 44.6 parts of 2-amino-4-acetamidophenetole (dried), dry product-NH 2 Value 99.27%, HPLC purity 99.89%, yield 91.9%.
Embodiment 3
[0024] Add 59.5 parts of 2-nitro-4-acetamido amphetamine to 240 parts of water (dried), add 5.0 parts of glacial acetic acid, and adjust the pH to 5 with baking soda; 24.5 parts of sodium hydrosulfide solution (100% reduction, solution concentration 20%) was added dropwise at a constant speed at 105°C. After the dropwise addition, keep the reaction at 100-105°C for 1 hour. Slowly lower the temperature to below 30°C to precipitate crystals, filter, wash the crystals with cold water until the filtrate pH = 7, and obtain 48.7 parts of 2-amino-4-acetylaminophenpropyl ether (dried), dry product-NH 2 Value 99.50%, HPLC purity 99.64%, yield 93.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com