Diene selective hydrogenation catalyst and preparation method
A hydrogenation catalyst and catalyst technology, applied in the field of selective hydrogenation of diolefins in pyrolysis gasoline and FCC gasoline fractions, can solve the problems of high reaction temperature, activity, selectivity and stability to be improved, and achieve raw material applicability Strong, good low temperature activity, high hydrogenation activity and hydrogenation selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
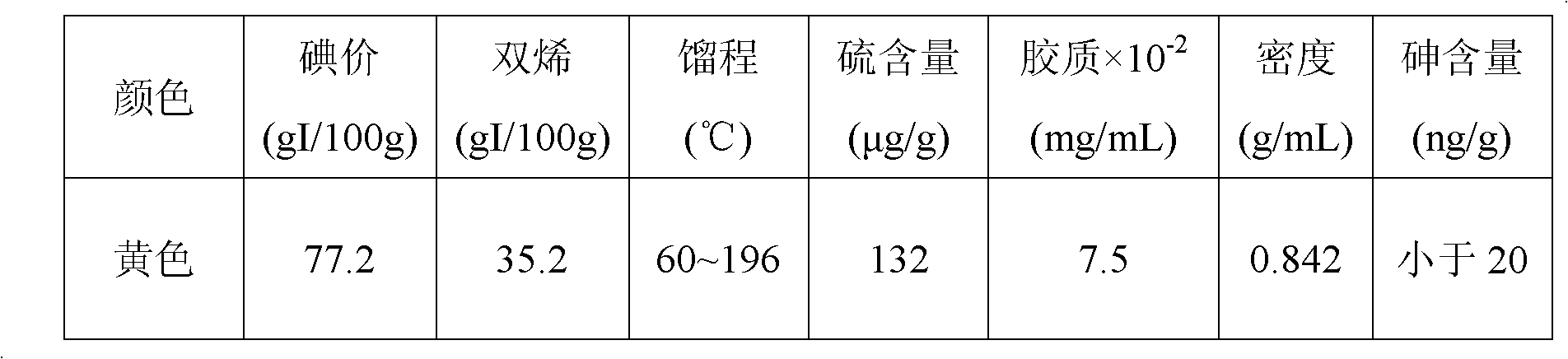
Examples
Embodiment 1
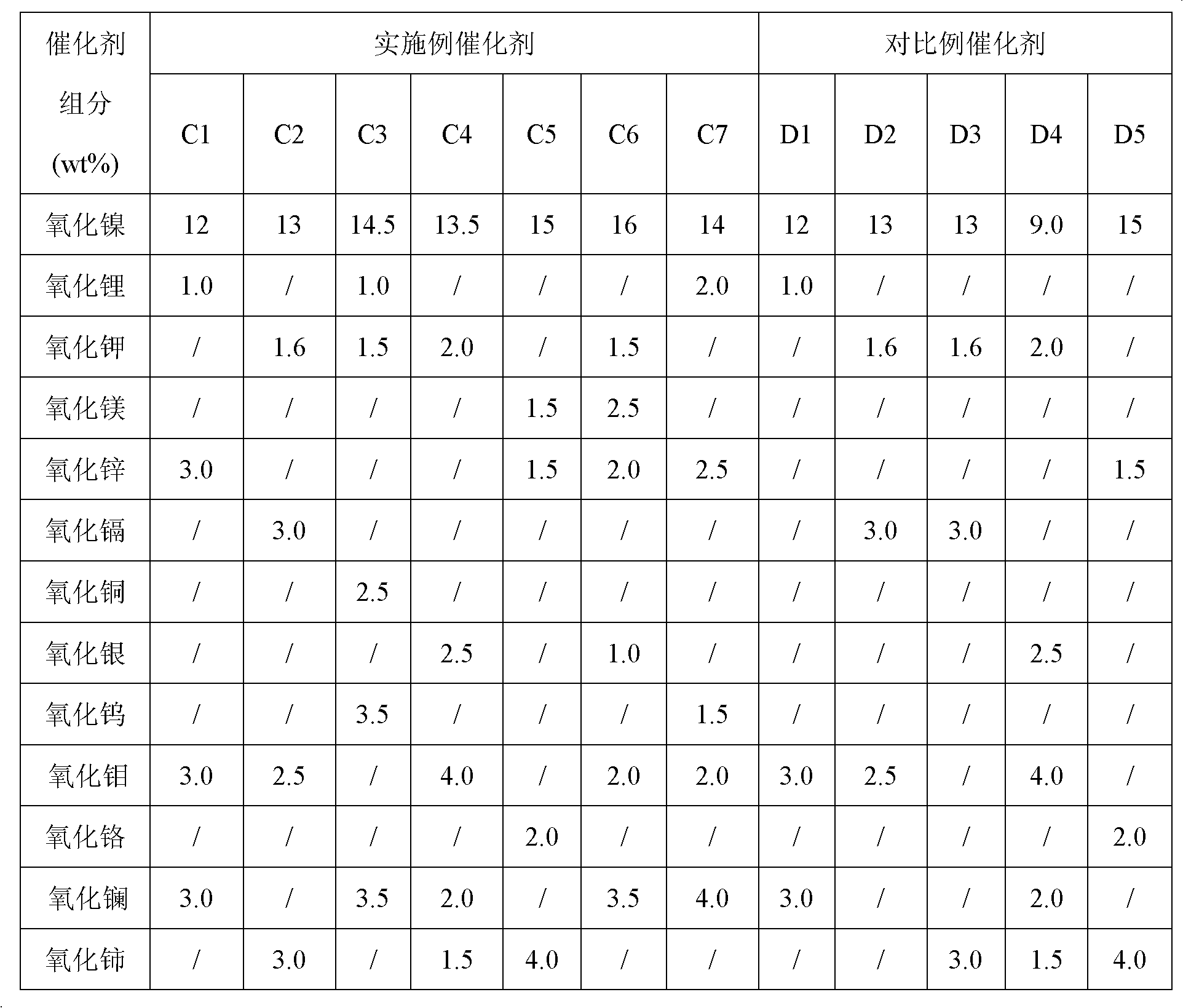
[0037] Pseudoboehmite, lithium nitrate solution, nitric acid and water were mixed and kneaded, extruded, dried at 120°C for 6 hours, and calcined at 900°C for 5 hours to obtain the catalyst carrier Z A . Prepare the mixed impregnation solution of nickel nitrate, zinc nitrate, ammonium molybdate, lanthanum nitrate, EDTA, triethylene glycol, wherein the molar ratio of EDTA, triethylene glycol and active component nickel is 1:5, and then Adjust the pH value of the impregnating solution to 4, impregnate in 100gZ AOn the carrier, aging for 12 hours, drying at 120°C, and roasting at 400°C for 4 hours, the finished product containing nickel oxide 12wt%, lithium oxide 1.0wt%, zinc oxide 3.0wt%, molybdenum oxide 3.0wt%, lanthanum oxide 3.0wt% Catalyst C1.
Embodiment 2
[0039] Pseudo-boehmite, nitric acid and water were mixed and kneaded, extruded, dried at 120°C for 6 hours, and calcined at 950°C for 5 hours to obtain the catalyst carrier Z B . Prepare a mixed impregnation solution of nickel nitrate, potassium nitrate, cadmium nitrate, ammonium molybdate, cerium nitrate, and triethylene glycol, wherein the molar ratio of triethylene glycol to the active component nickel is 1:7, adjust the impregnation Liquid pH value to 3, impregnated in 100gZ B On the carrier, aging for 12h, drying at 120°C, and roasting at 450°C for 4h, the finished product containing 13wt% of nickel oxide, 1.6wt% of potassium oxide, 3.0wt% of cadmium oxide, 2.5wt% of molybdenum oxide and 3.0wt% of cerium oxide was obtained Catalyst C2.
Embodiment 3
[0041] The carrier preparation method is the same as in Example 2. Prepare a mixed impregnation solution of nickel nitrate, lithium nitrate, potassium nitrate, copper nitrate, ammonium metatungstate, lanthanum nitrate, and citric acid, wherein the molar ratio of citric acid to the active component nickel is 1:8, and adjust the pH value of the impregnation solution to 4, impregnated in 100gZ B on the carrier, aged for 12 hours, dried at 120°C, and calcined at 400°C for 4 hours to obtain 14.5wt% nickel oxide, 1.0wt% lithium oxide, 1.5wt% potassium oxide, 2.5wt% copper oxide, 3.5wt% tungsten oxide, Lanthanum oxide 3.5wt% finished catalyst C3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com