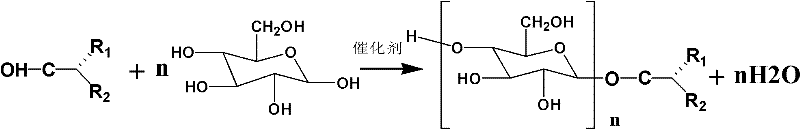
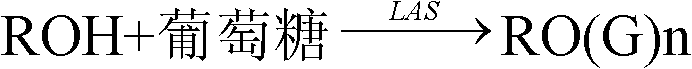Isomeric primary alcohol alkyl glycoside and synthesis method and application thereof
A technology of primary alcohol alkyl glycoside and synthesis method, which is applied in the field of fine chemical surfactants, can solve the problems of product purity and surface activity reduction, product odor and other problems, and achieve strong dispersion and decontamination capabilities, high added value, and side reactions little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] Example 1: Isomerized Primary Decyl Alkyl Glycosides
[0028] Add 1.14 moles of isomeric primary decanol and 0.32 moles of anhydrous glucose into the reaction kettle, add 0.58 grams of dodecylbenzenesulfonic acid, check the airtightness of the device and start to heat up, and the stirring speed is above 840r / min . Increase the temperature to 115°C (±2°C) within 20 minutes, and adjust the pressure to a residual pressure of 30mmHg. After reacting for 5.5 hours, the product was qualified, and the isomeric primary decanol alkyl glycoside was obtained through dealcoholization.
[0029] Finished product treatment: dealcoholization under pressure conditions of residual pressure less than 5mmHg and temperature conditions of 180°C, dissolving the product in deionized water to prepare a 51.0% solution, and then bleaching with 2% hydrogen peroxide relative to the mass of the alkyl glycoside solution , to obtain purified isomeric primary alcohol alkyl glycosides.
example 2
[0030] Example 2: Isomerized primary octanol alkyl glycosides
[0031] Add 1.47 moles of isomeric primary octanol and 0.28 moles of anhydrous glucose into the reaction kettle, add 0.5 grams of dodecylbenzenesulfonic acid, check the airtightness of the device and start to heat up, and the stirring speed is above 840r / min . The temperature was raised to 113°C (± 2°C) within 20 minutes. Adjust the pressure to a residual pressure of 40mmHg. After 5 hours of reaction, the product is qualified, and the isomeric primary octanol alkyl glycoside is obtained through dealcoholization.
[0032] Finished product treatment: dealcoholization under pressure conditions of residual pressure less than 5mmHg and temperature conditions of 150°C, dissolving the product in deionized water to prepare a 51.0% solution, and then bleaching with 1.5% hydrogen peroxide relative to the mass of the alkyl glycoside solution , to obtain purified isomeric primary alcohol alkyl glycosides.
example 3
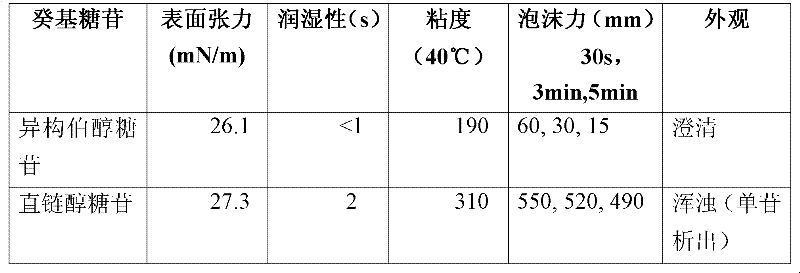
[0033] Example 3: The performance experiment of the isomeric primary alcohol alkyl glycoside synthesized in embodiment 1 and 2
[0034] Prepare the straight-chain alkyl glycosides with the same carbon number as in Examples 1 and 2, and the reaction process is as follows:
[0035]
[0036] In the above formula, R represents a C6-18 alkyl group, G represents the number of sugar units, and n represents the number of sugar units combined on each alkyl group or the average degree of polymerization.
[0037] LAS is dodecylbenzenesulfonic acid.
[0038] Surface tension (mN / m), see GB / T 22237-2008 for experimental methods
[0039] Wettability (s), see GB / 11983-2008 for test method
[0040] Viscosity (mPa.s), see GB / 15357-94 for the experimental method (usually the measurement temperature is 20°C, when the viscosity is greater than 10000mPa.s, the measurement temperature should be raised to 40°C)
[0041] Foam force (mm) 30s, 3min, 5min, see GB / T 7462-94 for the test method
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com