Catalyst for preparing caprolactam from cyclohexanone-oxime by liquid-phase Beckmann rearrangement
A technology of Beckmann rearrangement and cyclohexanone oxime, which is applied in the field of caprolactam preparation, can solve the problems of complex preparation process and difficult recovery, and achieve the effect of low reaction temperature and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
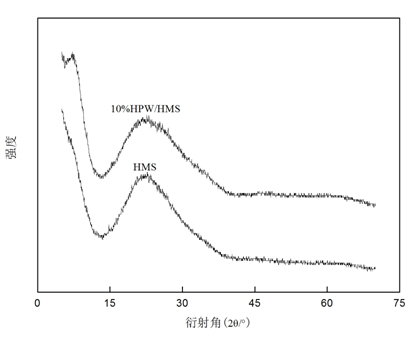
[0030] Mesoporous molecular sieve HMS supported phosphotungstic heteropolyacid HPW (HPW / HMS) catalyst was prepared by equal volume impregnation method: Weigh 0.2 g phosphotungstic heteropolyacid (H 3 PW 12 o 40 ·xH 2 O) Dissolve in 10 ml deionized water to obtain a clear solution. Take 2.0 g of HMS and place it in the above clear solution, and let it stand at room temperature for 24 h. The white solid powder was obtained by rotary evaporation at 60°C, and calcined at 180°C for 9 h to obtain HPW / HMS with a loading of HPW of 10 wt.%.
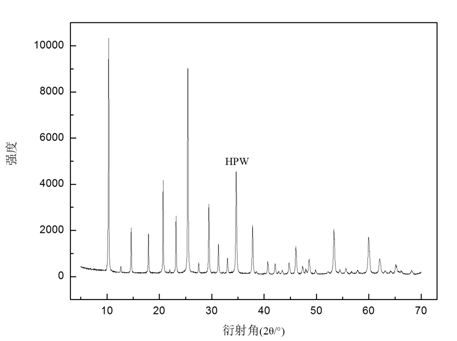
[0031] in the attached figure 1 Among them, HPW has two obvious characteristic diffraction peaks at 2θ=10.3° and 25.4°, indicating that the HPW used in the experiment has a Keggin structure, and the diffraction intensity of these two characteristic peaks is very strong, and the peak shape is sharper, indicating that the HPW has a Keggin structure. The degree of crystallinity is very high, and the crystal form is relatively perfect.
[0032] ...
Embodiment 2~5
[0036] The preparation of the HPW / HMS with a load of 20wt.%, 30wt.%, 40wt.% and 50wt.% is the same as in Example 1, except that phosphotungstic heteropolyacid (H 3 PW 12 o 40 ·xH 2 O) have masses of 0.4 g, 0.6 g, 0.8 g and 1.0 g, respectively.
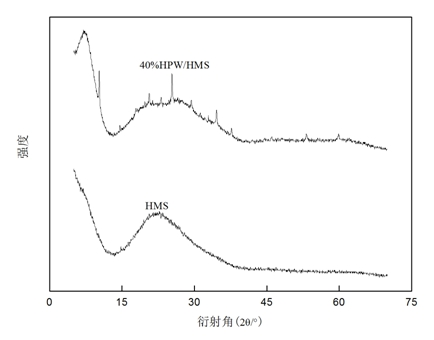
[0037] in the attached image 3 Among them, when the loading of HPW is 40wt.%, the characteristic diffraction peaks of HPW appear in many places such as 2θ=10° and 25°, and the peak shape of these characteristic diffraction peaks is sharp and the peak intensity is strong. XRD technology The existence of HPW crystals was detected by means, indicating that when the loading of HPW increased to 40wt.%, aggregation began to occur on the surface of HMS to form HPW crystals.
[0038] The activity test operation steps and reaction conditions of the catalyst are the same as in Example 1. The experimental results are shown in Table 1.
[0039] Table 1 Effect of HPW loading on Beckmann rearrangement of cyclohexanone oxime
[0040] ...
Embodiment 6~7
[0042] The preparation steps and reaction conditions of the catalyst in Example 2 are the same, except that the calcination temperatures of the supported catalyst HPW / HMS are 240° C. and 300° C. respectively. The experimental results are shown in Table 2.
[0043]
[0044] Table 2 Effect of calcination temperature of HPW / HMS on the Beckmann rearrangement reaction of cyclohexanone oxime
[0045] Example Calcination temperature (°C) Yield of caprolactam (%) Selectivity of caprolactam (%) 2 180 44.2 82.3 6 240 55.2 84.1 7 300 55.5 78.0
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com