Layered double hydroxides (LDHs) loading highly-dispersed fullerenes and preparing method of LDHs
A technology of hydrotalcite and fullerene, applied in the field of hydrotalcite material loaded with highly dispersed fullerene and its preparation, to achieve the effect of stable intercalation structure, strong interaction force and easy realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
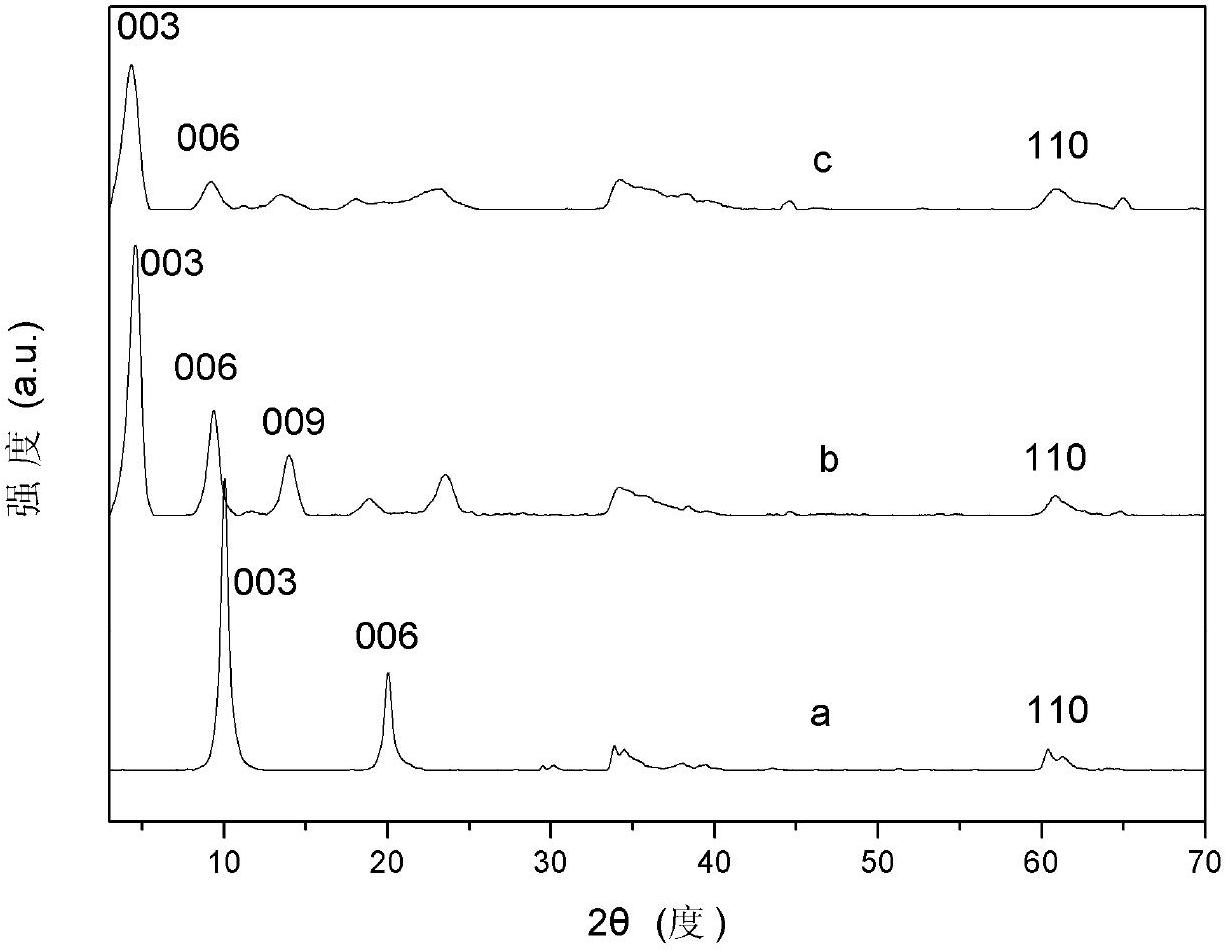
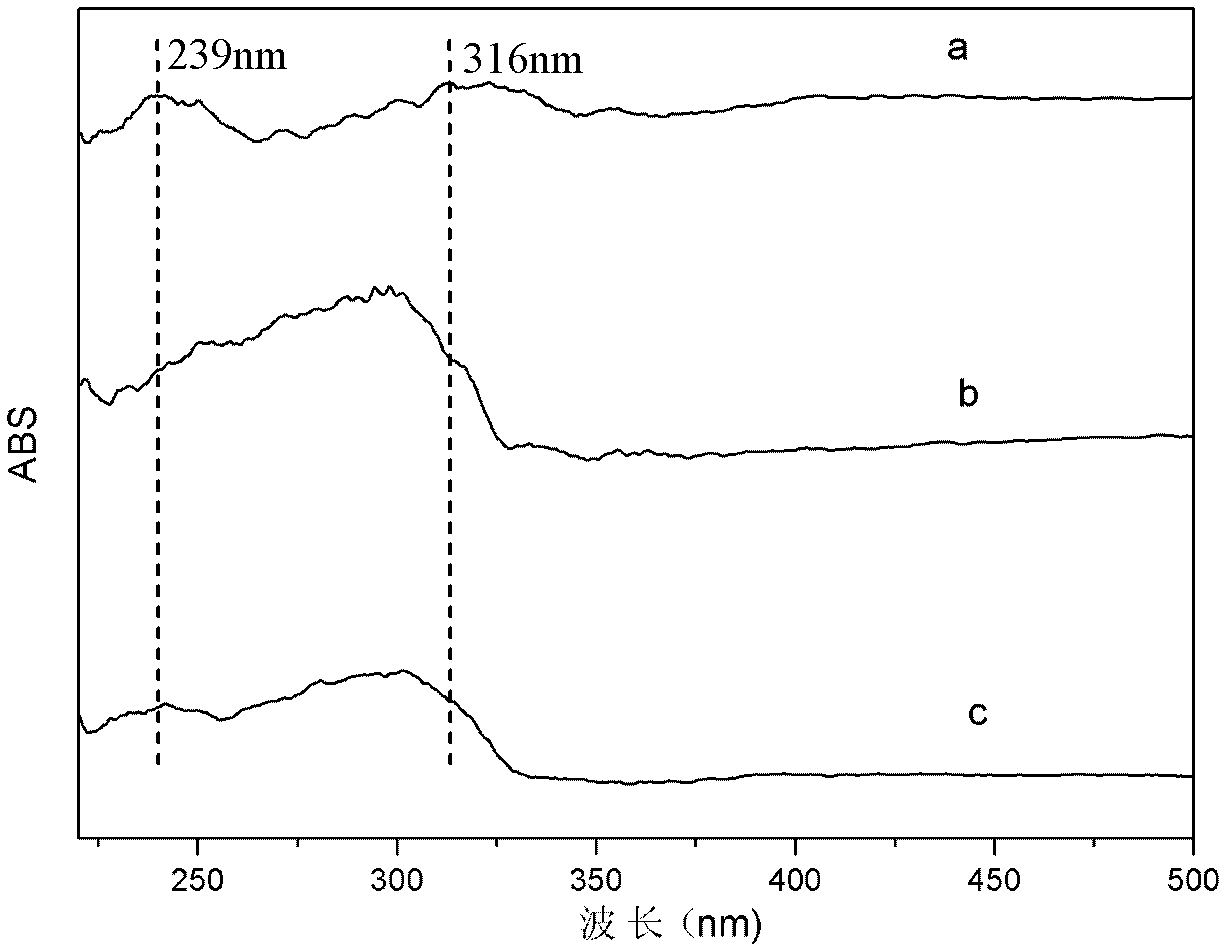
[0039] Step A: take by weighing 17.8g Zn(NO 3 ) 2 ·6H 2 O, 11.3g Al(NO 3 ) 3 9H 2 O dissolved in 100ml to remove CO 2 Mixed salt solution was prepared with water, another 5.6g NaOH was dissolved in 50ml to remove CO 2 Prepare alkaline solution in water, N at room temperature 2 Protection Use the double-drop method to add the salt solution and the alkali solution into the four-neck flask, and stir vigorously. Adjust the pH value to 7 with 0.1mol / L NaOH solution. The resulting slurry was crystallized at 60°C for 72 hours, and the product was placed in a centrifuge at a speed of 2500 rpm for 5 minutes, and then used to remove CO 2 Wash with water until neutral; take out the sample and dry it at 50°C for 48 hours for characterization, and get ZnAl-NO 3 -LDHs, whose Zn 2+ / Al 3+ =2.
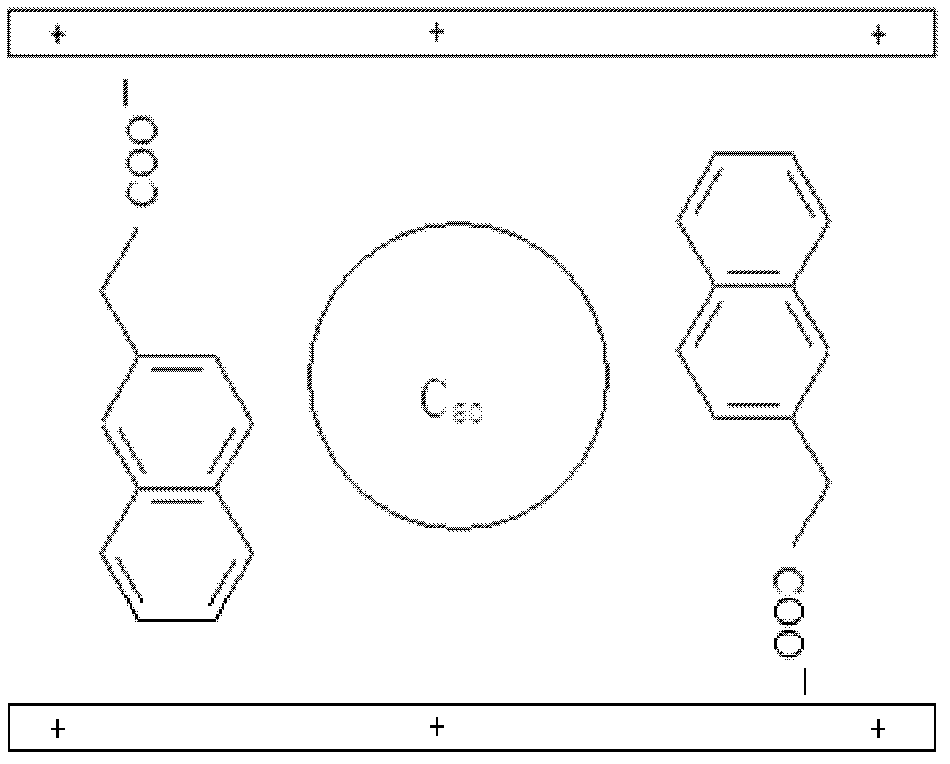
[0040] Step B: the sodium salt of 10.9g naphthaleneacetic acid [C 12 h 9 o 2 Na] solid dissolved in 100mL to remove CO 2 After the deionized water was transferred into the four-necked ...
Embodiment 2
[0044] Step A: take by weighing 15.4g Mg(NO 3 ) 2 ·6H 2 O and 11.3g Al(NO 3 ) 3 9H 2 O dissolved in 100ml to remove CO 2 Mixed salt solution was prepared with water, another 2.0g NaOH was dissolved in 50ml to remove CO 2 Prepare alkaline solution in water, N at room temperature 2 The salt solution and alkali solution are added into the four-necked bottle by the double-drop method, and the pH value is adjusted to 10 with 5mol / L NaOH solution after the drop is completed. The resulting slurry is crystallized at 90°C for 12 hours, and the product is put into a centrifuge at a speed of 3500 rpm. Centrifuge at 1 / min for 3min, then use to remove CO 2 Wash with water until neutral; take out a small amount of samples and dry them at 90°C for 20 hours for characterization to obtain MgAl-NO 3 -LDHs, its Mg 2+ / Al 3+ =2.
[0045] Step B: the sodium salt of 10.9g naphthaleneacetic acid [C 12 h 9 o 2 Na] solid dissolved in 100mL to remove CO 2 After the deionized water was tr...
Embodiment 3
[0049] Step A: Weigh 17.5g Co(NO 3 ) 2 ·6H 2 O, 11.3g Al(NO 3 ) 3 9H 2 O dissolved in 100ml to remove CO 2 Mixed salt solution was prepared with water, another 8.0g NaOH was dissolved in 50ml to remove CO 2 Prepare alkaline solution in water, N at room temperature 2 Protection Use the double-drop method to add the salt solution and the alkali solution into the four-neck flask, and stir vigorously. Adjust the pH value to 7 with 2.0mol / L NaOH solution. The resulting slurry was crystallized at 70°C for 36 hours, and the product was placed in a centrifuge at a speed of 3000 rpm for 4 minutes, and then used to remove CO 2 Wash with water until neutral; take out the sample and dry it at 60°C for 36 hours for characterization, and obtain CoAl-NO 3 -LDHs, whose Co 2+ / Al 3+ =2.
[0050] Step B: the sodium salt of 10.9g naphthaleneacetic acid [C 12 h 9 o 2 Na] solid dissolved in 100mL to remove CO 2 After the deionized water was transferred into the four-necked bottle, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com