Substrate with photo-controllable cell adhesion property, method for analyzing and fractionating cells, and device for analysis and fractionation of cells
An adhesive material and adhesive technology, applied in the field of stem cell research and regenerative medicine, can solve the problems of reduced survival rate, fluorescence correction, complex optical axis adjustment, cell damage, etc., and achieve the effect of improving purity and simple real-time operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
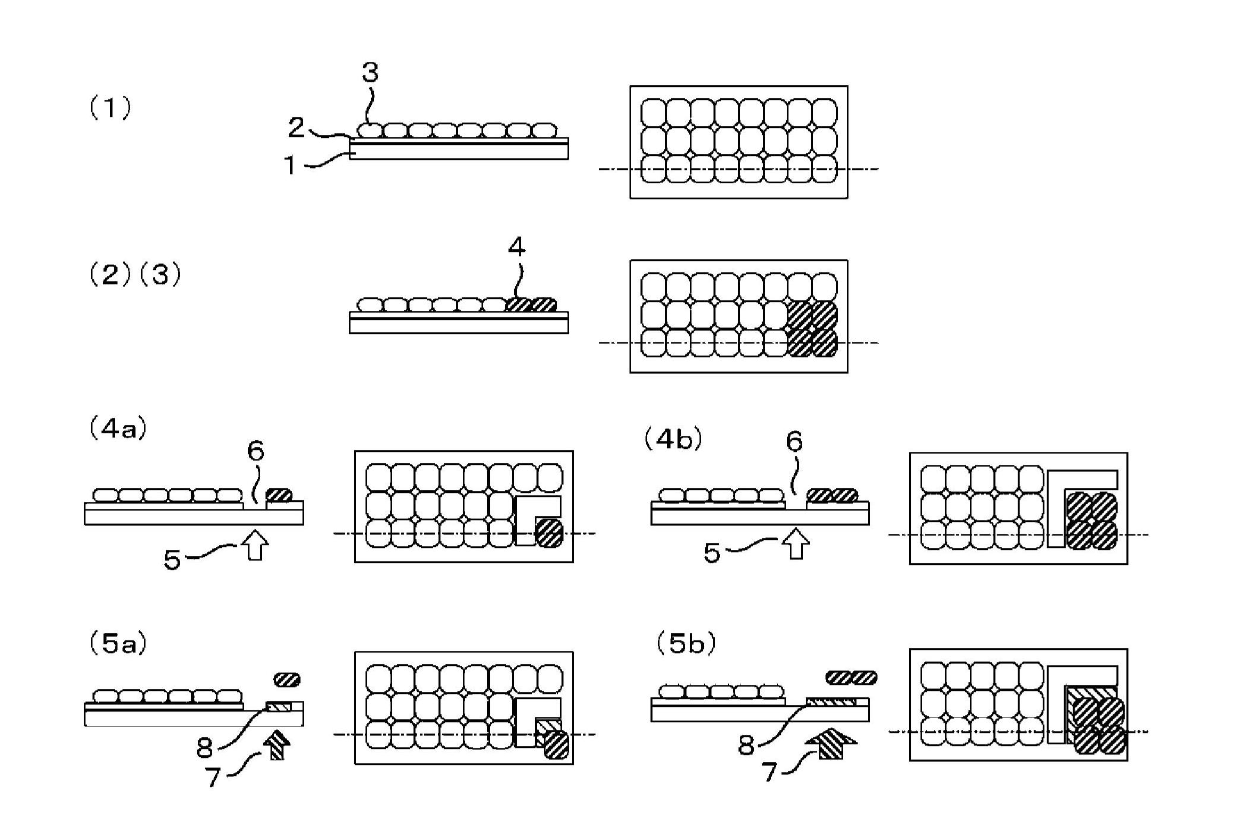
[0112] Prepare the methacrylic polymer represented by the general formula (1) (R 1 :Methyl group, n:1) 20 mol%, methacrylic polymer represented by general formula (2) (R 1 : Methyl, R 2 : Butylene) 50 mol% and the methacrylic polymer represented by the general formula (10) (R 1 : Methyl, R 6 : OCOCH 2 OCH 2 CH 2 OCH 2 CH 2 O, R 4 : Br, X: CH 2 CO 2 H, n: 1) 30 mol% terpolymer (molecular weight: 5,000 to 50,000) membrane glass culture vessel. Add a cell suspension of human bone marrow stromal cells and human adipocyte differentiation medium (Cell Applications) to it, 2 Culture in an incubator. At the stage when it became 40% full, the glass culture container was installed in the apparatus of the present invention, and the light of 450 nm or less was blocked for microscopic observation. Use the monitor to confirm the position of the cells considered to be abnormal, and set the periphery of the abnormal cell group as the laser ablation area (refer to figure 1 (1)(2)(3)(4a)(5a)). P...
Embodiment 2
[0115] After continuing the culture of the sample of Example 1, the glass culture container was taken out of the incubator. The cells were washed with PBS and treated with a blocking solution for cell surface labeling (JRH) for 1 hour. In order to detect the mesenchymal stem cell marker: CD105, a cell surface labeling blocking solution dilution solution of mouse anti-human CD105 antibody (abcam) was added, and the reaction was carried out at room temperature for 1 hour. After washing with PBS, the cell surface labeling blocking solution diluent of Alexa Fluor 488 labeled anti-mouse IgG antibody (Invitrogen) was added, and the reaction was light-shielded for 1 hour. After the reaction, the solution was replaced with PBS. Next, in order to detect fat cells, OilRed O staining solution (Sigma) was added and left at room temperature for 1 hour for staining, and the solution was replaced with PBS. The observation and differentiation of the cells are performed as follows. A glass c...
Embodiment 3
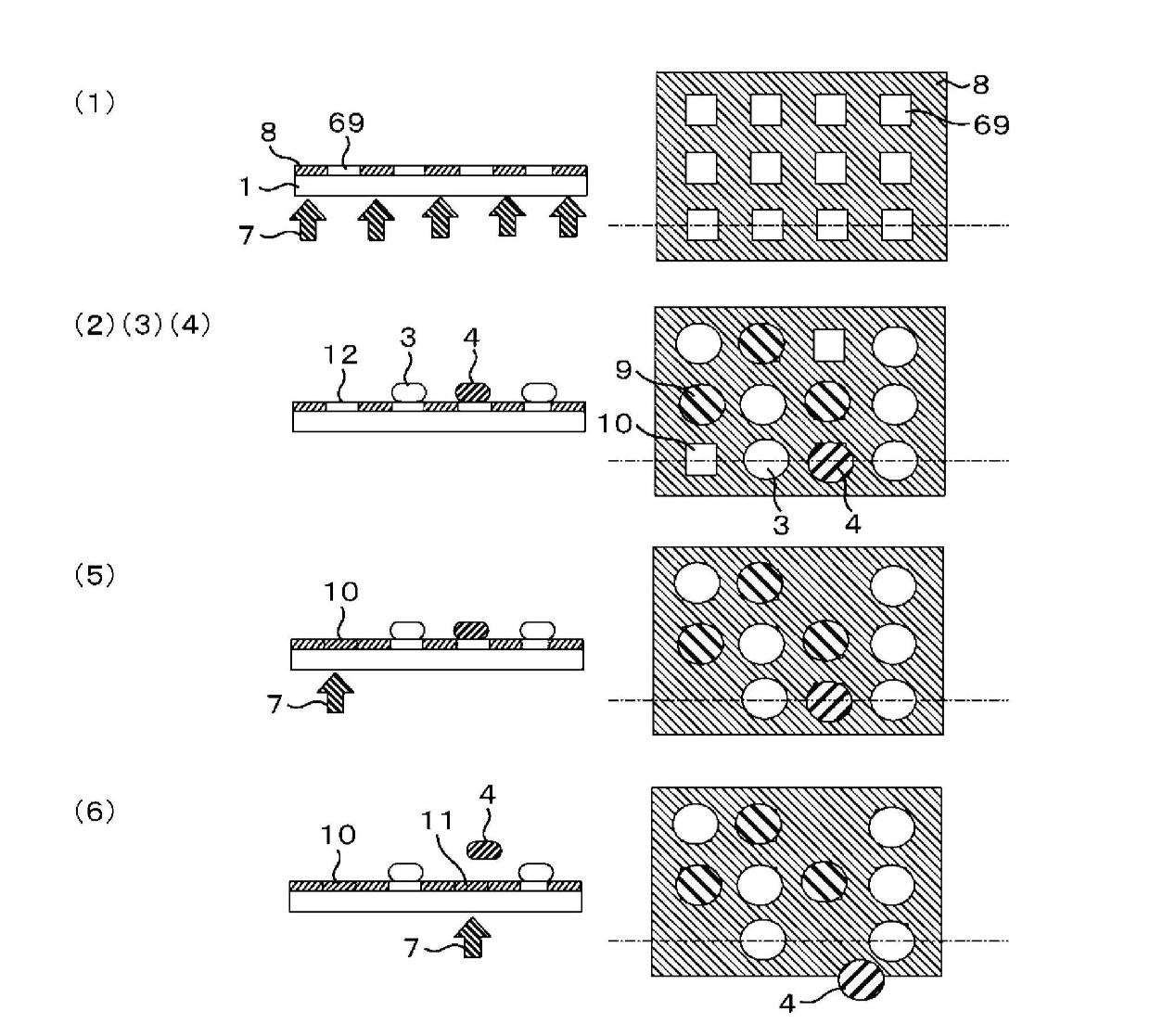
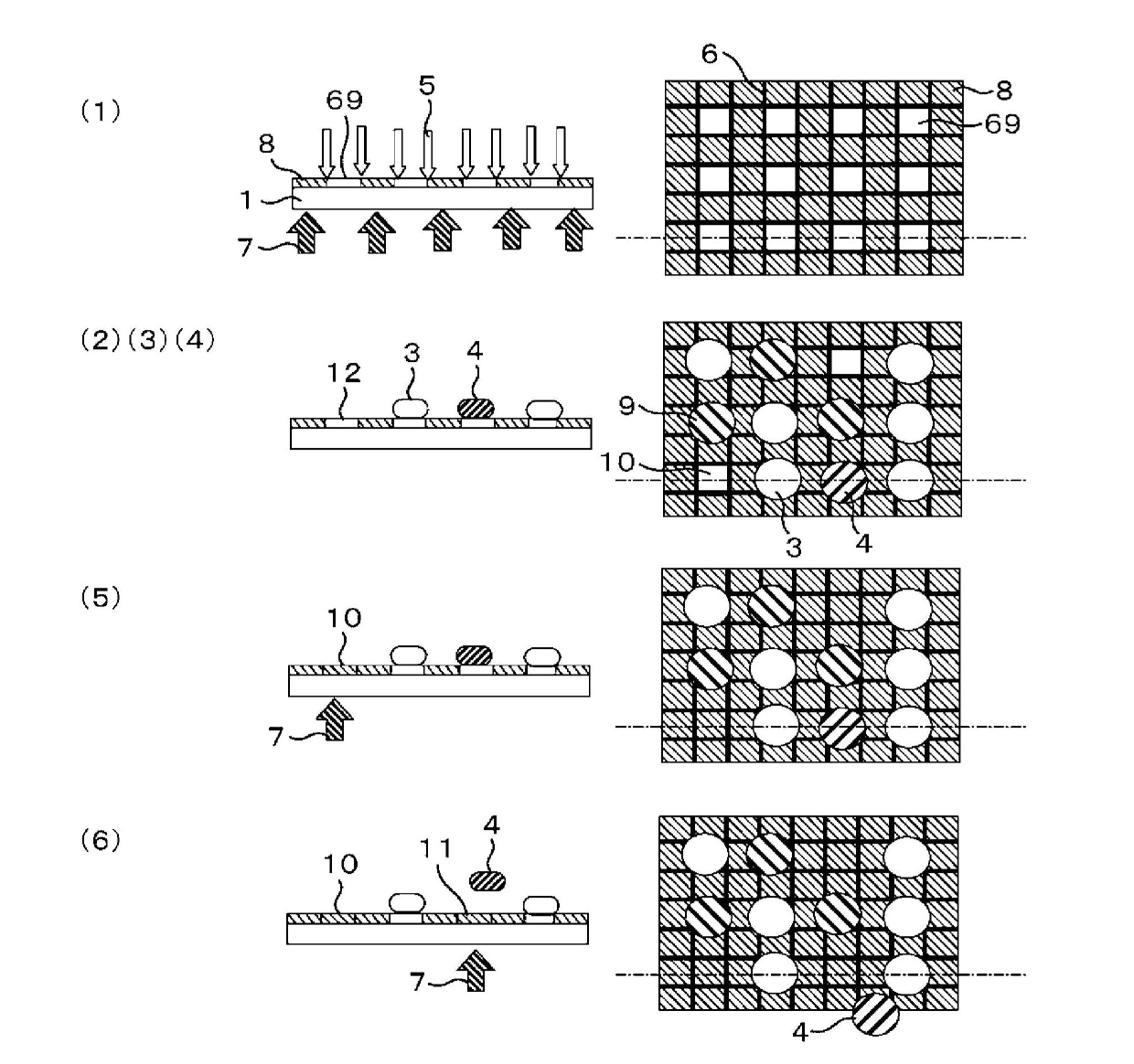
[0118] HBSS was added to another glass culture container cultured in the same manner as in Example 2 and washed, and a Trypsin / EDTA solution was added, and it was left at room temperature for several minutes to peel the cells from the glass culture container. Then, the tripsin neutralization solution was added to stop the reaction, the cells were recovered into a centrifuge tube by flushing, and centrifuged for a few minutes, the supernatant was removed, and the culture medium was added to make a cell suspension. In addition, the application of mesenchymal stem cell markers and adipocyte staining were also performed in the same manner as in Example 2. Next, prepare an alkoxysilane represented by general formula (11) (R 2 : (CH 2 CH 2 O) 2 CH 2 CH 2 , R 3 : Methyl, R 4 : Br, R 5 : O, R 6 : OCOCH 2 O(CH 2 CH 2 O) 3 , X: CH 2 NH 2 ) A membrane glass culture vessel with PBS added and set in the device of the present invention. Then, the cell adhesion areas of 70 μm square are arran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com