Application of LECT2 protein in preparation of antiviral drugs
An antiviral drug, LECT2 technology, applied in antiviral agents, peptide/protein components, pharmaceutical formulations, etc., to achieve the effects of improving macrophage activity, inhibiting spleen enlargement, and inhibiting replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
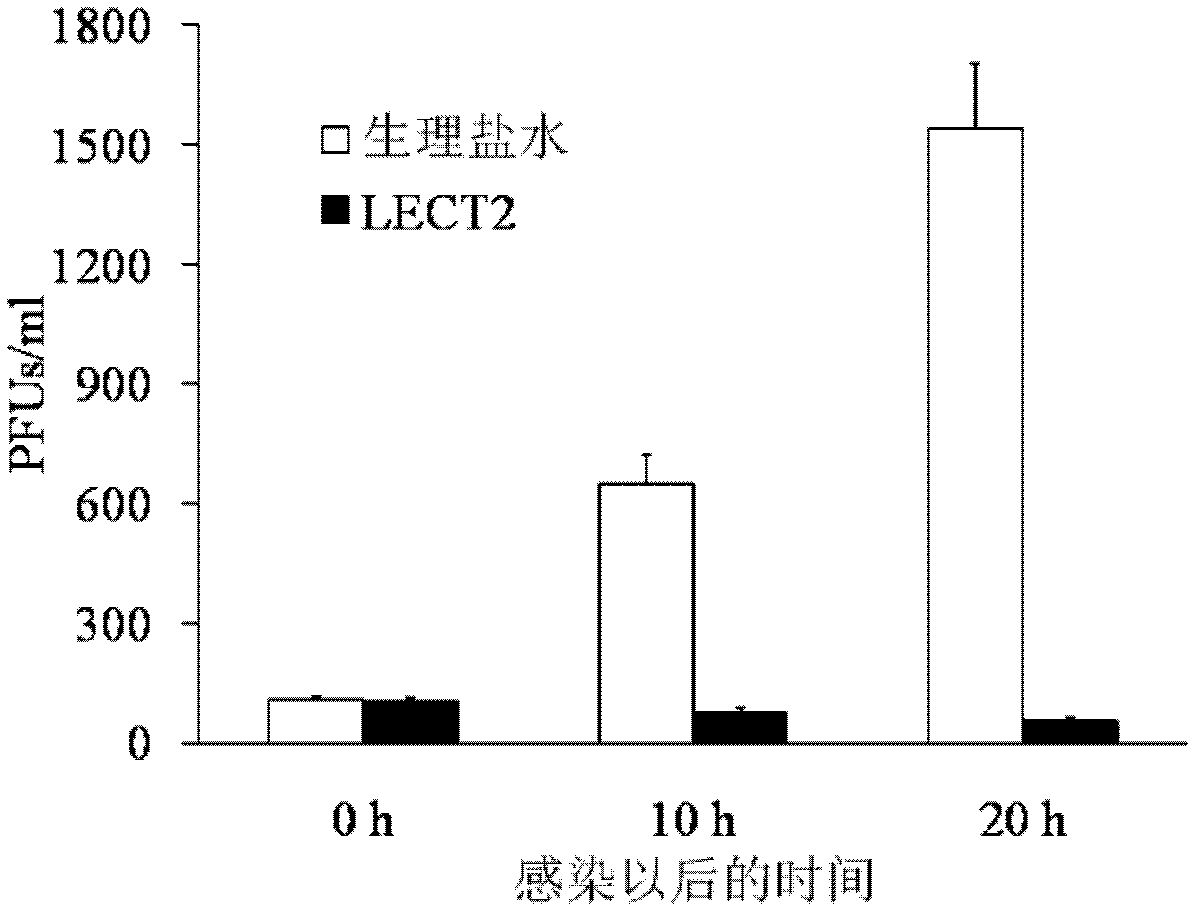
[0017] 1.1 Materials: Influenza A virus strain (H1N1, A / Puerto Rico / 8 / 1934) was purchased from Zhejiang Academy of Medical Sciences, 9-day-old SPF (specific pathogen-free) chicken embryos were inoculated after virus recovery, and infected chicken embryos were harvested after 72 hours of culture The allantoic fluid was used to detect the hemagglutination titer of the virus, and the SPF chicken embryos were transferred to the 2nd to 3rd generations and stored at -70°C. The LECT2 protein is a recombinant LECT2 protein, and the preparation of the recombinant LECT2 protein can be carried out according to the methods published by Ovejero and others, which will not be described here.
[0018] 1.2 Recombinant LECT2 protein promotes IFN-γ secretion of macrophages
[0019] 1.2.1 Culture of lung macrophages: mice were anesthetized and fixed, the neck was shaved and disinfected, the neck was cut in the middle, and the trachea was exposed and stripped. Thread a slipknot at the proximal en...
Embodiment 2
[0024] Example 2 is basically the same as Example 1, except that the recombinant LECT2 protein is replaced by the LECT2 protein extracted directly from animals. Effect. The H1N1 virus can also be replaced by other Orthomyxoviridae members to obtain the above effects.
Embodiment 3
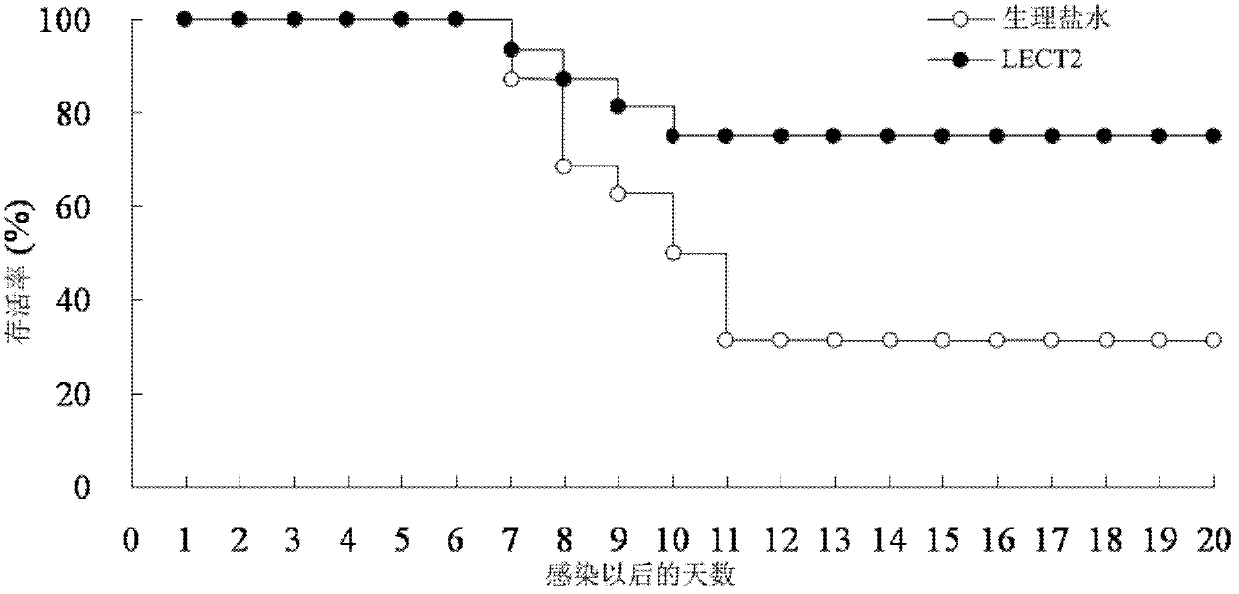
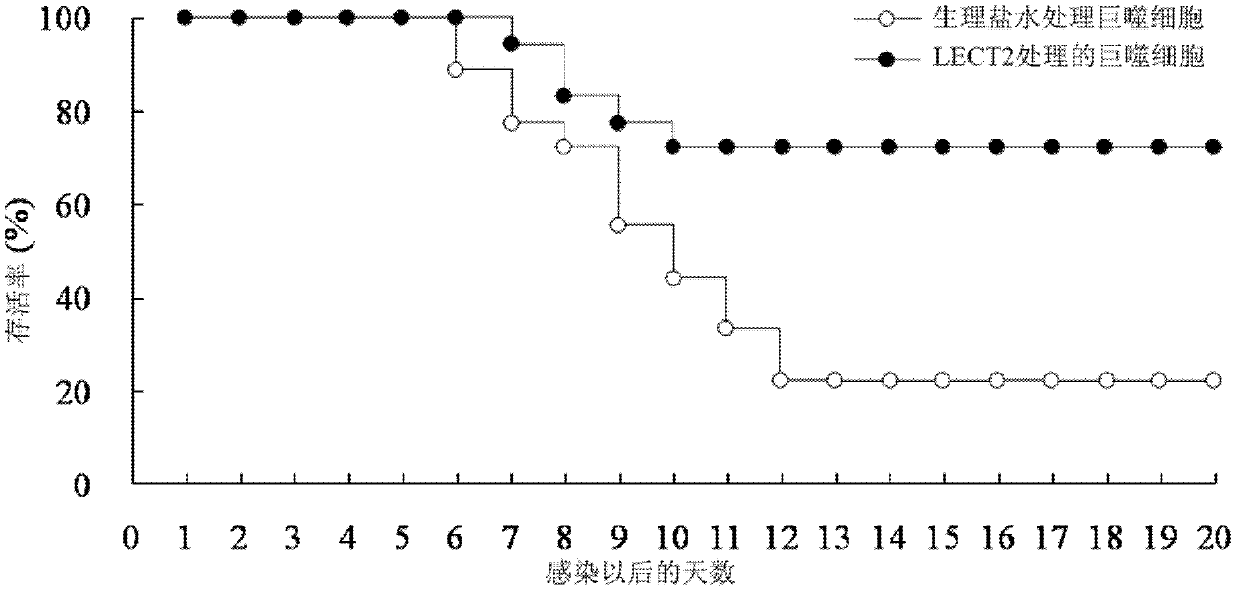
[0026] 2.1 LECT2 protein treatment inhibits mouse spleen enlargement caused by murine leukemia virus (MuLV, where Friend type is abbreviated as F-MLV) infection: F-MLV is quoted from the American Classical Culture Collection, and passed in vivo in mice to produce 10% spleen suspension, stored at -70°C. And determine the virus titer PFU. The mice were randomly divided into normal group (without injection of F-MLV+normal saline), control group (injection of F-MLV+normal saline), and experimental group (injection of F-MLV+LECT2), with 12 mice in each group Except for the normal group, the injection volume of F-MLV was 0.5ml of virus liquid (virus titer 100PFU) by intraperitoneal injection for each mouse. The experimental group was intraperitoneally injected with pure LECT2 protein (0.2 μg / g) 1 hour after F-MLV injection, and the normal group and control group were replaced with the same dose of normal saline for LECT2 protein. 30 days after the injection of F-MLV, the mice were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com