Cyclic phenyl phosphonate compound and method for preparing same
A technology for phenylphosphonates and compounds, applied in the field of compound synthesis, can solve the problems of limited expansion, easy solubility of flame retardants in water, low thermal stability of flame retardants, etc., and achieves improved thermal stability and reaction steps. And the requirements of the reaction equipment are simple and inexpensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
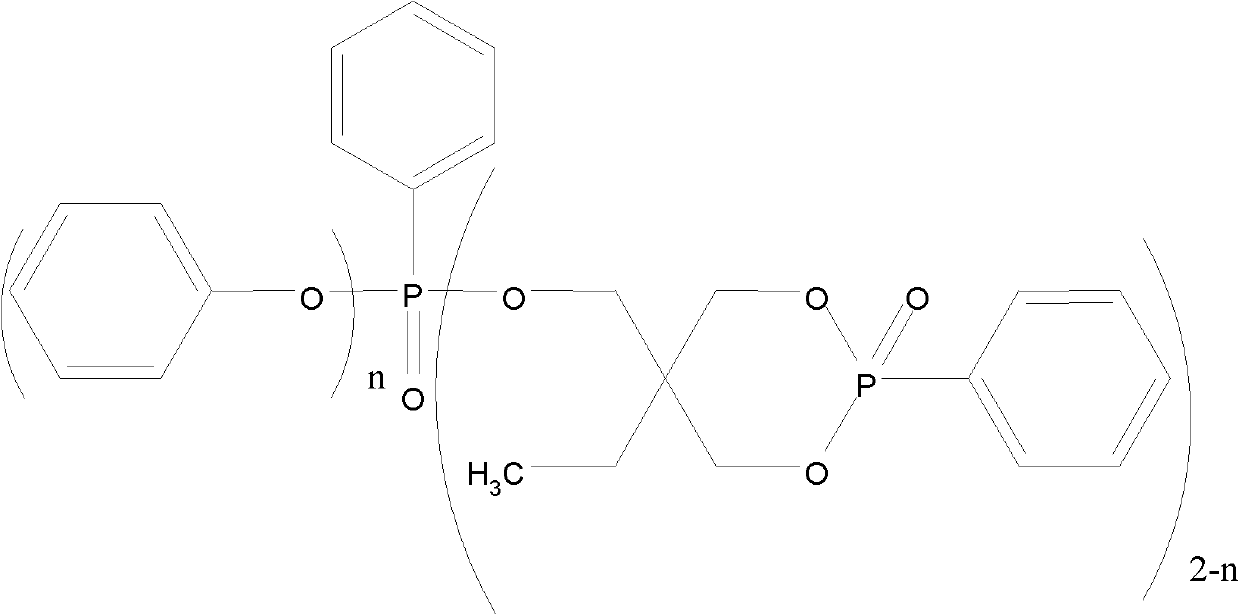
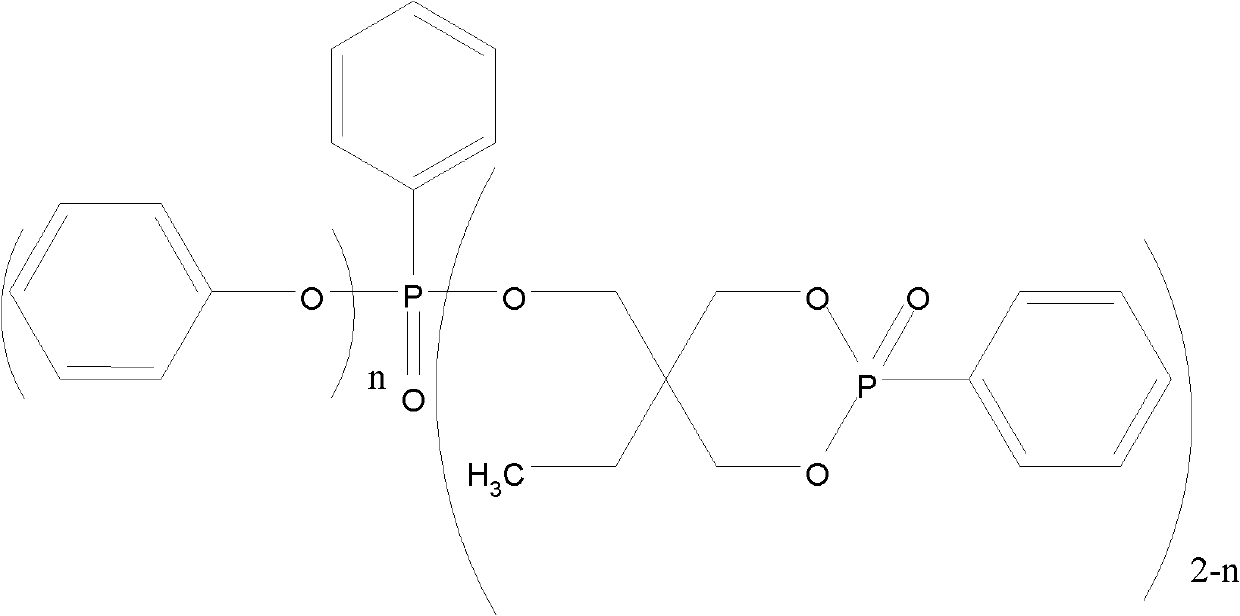
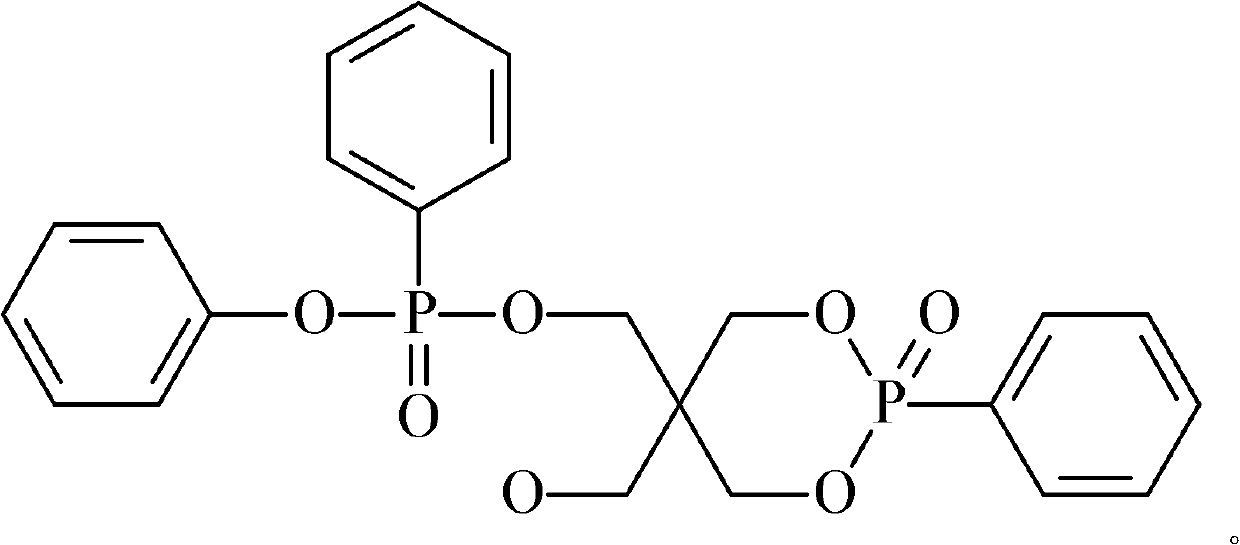
[0019] Add 134.17 g of trimethylolpropane, 124.08 g of trimethyl phosphite, and 2.5 g of triethylamine into a four-necked flask equipped with a stirrer, an atmospheric distillation device, a thermometer, and an air duct, and heat and stir to raise the temperature to 60°C , after the reaction time was 8 hours, rectification obtained 140.37g cage-shaped phosphoric acid ester intermediate. In the second step, 293.67g of triphenyl phosphite was added to the intermediate, and it was heated up to 150°C. After 15 hours of reaction, 376.60g of cyclic phenylphosphonate compounds were obtained. The reaction yield was 90%. The product structure is as follows:
[0020]
Embodiment 2
[0022] Add 134.17g of trimethylolpropane, 136.49g of trimethyl phosphite, and 0.8g of tetramethylammonium chloride into a four-necked flask equipped with a stirrer, an atmospheric distillation device, a thermometer, and an air duct, and heat and stir Raise the temperature to 70°C, the reaction time is 8 hours, and rectify to obtain 143.49g of the intermediate cage-shaped phosphate. In the second step, add 300.19g of triphenyl phosphite to the intermediate, and raise its temperature to 160°C, and the reaction time is After 16 hours, obtain 389.25g cyclic phenyl phosphonate compound, its productive rate is 91%, the chemical structural formula that obtains product is as follows;
[0023]
Embodiment 3
[0025] Add 134.17 g of trimethylolpropane and 148.90 g of trimethyl phosphite in a four-necked flask equipped with a stirrer, an atmospheric distillation device, a thermometer and an air duct, add 2 g of anhydrous aluminum trichloride, heat and stir to 80°C, the reaction time is 9 hours, rectification to obtain 155.97g of the intermediate cage-shaped phosphate ester. In the second step, 326.29g of triphenyl phosphite is added to the intermediate, and the mixture is heated to 170°C, and the reaction time is 16 Hour, obtain 437.04g cyclic phenyl phosphonate compound, its productive rate is 94%, obtain the chemical structure of product as follows:
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com