High coupling ratio holoantigen synthesis method of olaquindox residue marker
A synthesis method and whole antigen technology, applied in the field of immunochemical analysis, can solve the problems of low accuracy, inflammation, irritation, etc., and achieve the effect of less allergies, high safety, and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
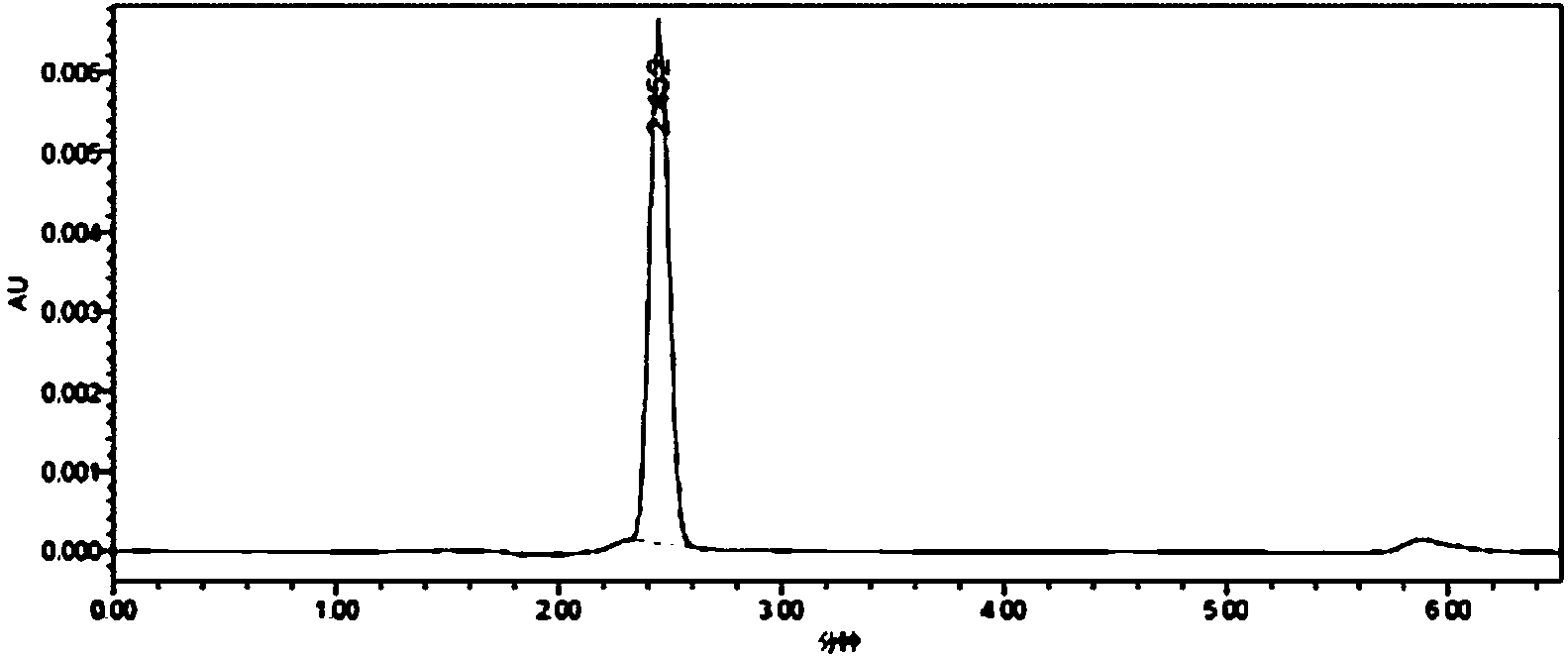
[0029] Synthesis of whole antigen (MQCA-BSA): Dissolve 25mmol of 3-methylquinoxaline-2-carboxylic acid (MQCA) in 0.5mL of dimethylformamide, then add 25mmol of N,N'-di Isopropylcarbodiimide (DIC), stirred for 30min, then added 40mmol of N-hydroxysuccinimide, stirred at 37°C in the dark for 16h, centrifuged to remove the precipitate, and the supernatant obtained by centrifugation was reaction solution A; 3.0×10 -4 Mmol bovine serum albumin (BSA, molecular weight 68000) was dissolved in 1.5mL of 0.02 mol / L phosphate buffer solution (pH=7.4) to obtain solution B; Stir overnight, place the reaction solution in an ultrafiltration tube (molecular cut-off 30 KD), centrifuge for 10 min, add 1 mL of 0.2 mol / L PBS buffer solution with pH=7.4, then centrifuge, repeat the operation 3 to 4 times, fully Wash off the unreacted small molecules, collect the filtrate, take 1mL of the filtrate to pass through a 0.22μm filter membrane, and use high-performance liquid chromatography to detect the...
Embodiment 2
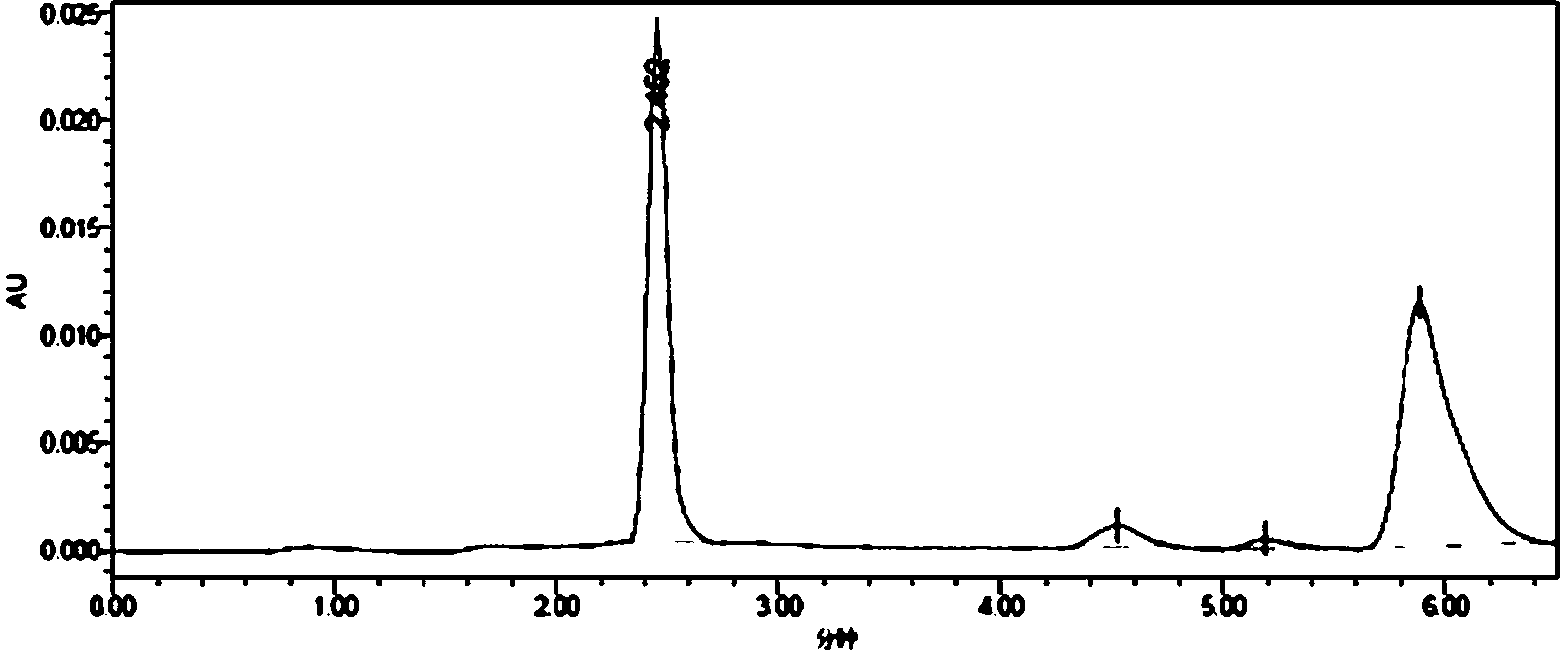
[0031] Synthesis of whole antigen (MQCA-OVA): Dissolve 25mmol of MQCA in 0.5mL of dimethylformamide, then add 25mmol of DIC, stir for 30min, then add 40mmol of N-hydroxysuccinimide, and avoid Stir the reaction for 16 hours, centrifuge to remove the precipitate, and the supernatant obtained by centrifugation is the reaction solution A; 4.4×10 -4 Dissolve mmol ovalbumin (OVA, molecular weight 45,000) in 1.5mL 0.02 mol / L phosphate buffer solution (pH=7.4) to obtain solution B; add solution B dropwise to reaction solution A, and stir at 37°C Overnight, put the reaction solution in an ultrafiltration tube (molecular cut-off: 30 KD), centrifuge for 10 minutes, add 1 mL of 0.2mol / L PBS buffer solution with pH=7.4, centrifuge again, repeat the operation 3 to 4 times, and fully wash away Collect the filtrate for uncompletely reacted small molecular substances, take 1mL of the filtrate to pass through a 0.22μm filter membrane, and use high-performance liquid chromatography to detect the...
Embodiment 3
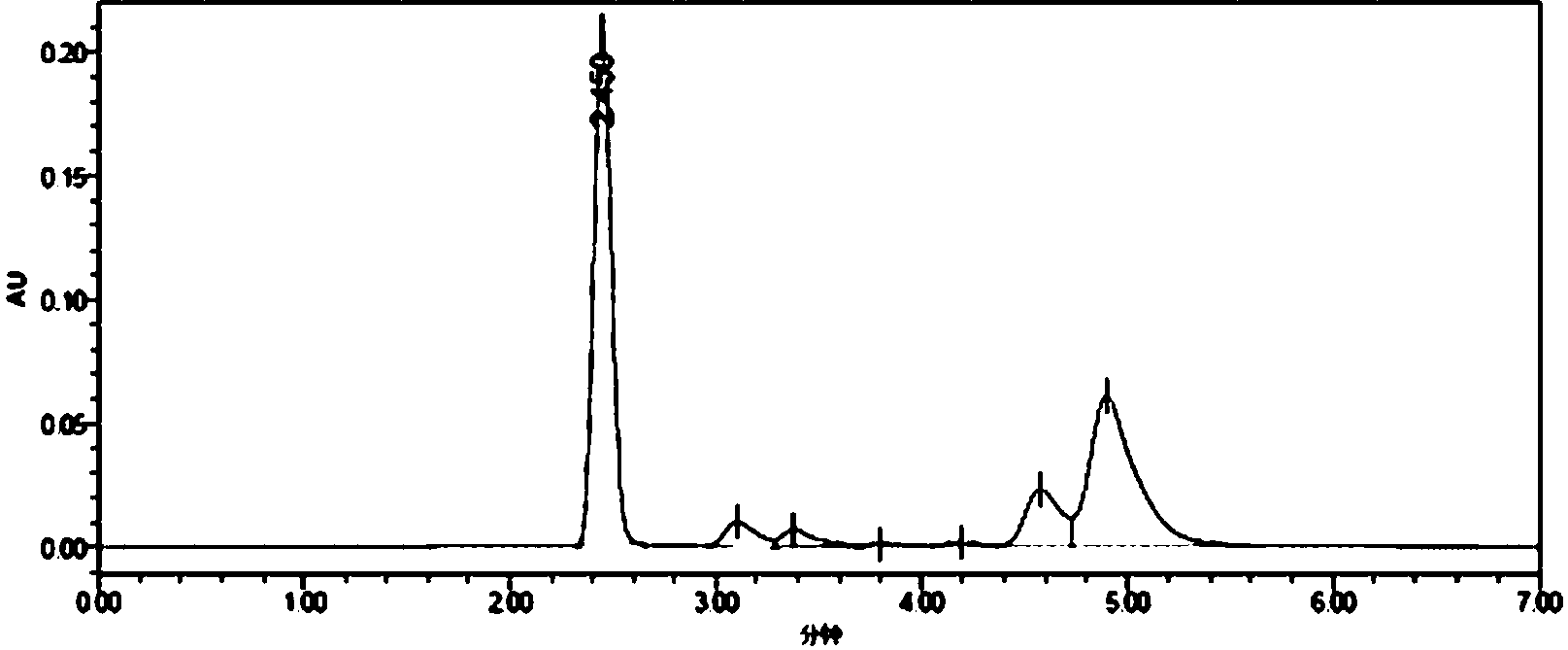
[0033] Synthesis of the whole antigen (MQCA-KLH): Dissolve 25mmol of MQCA in 0.5mL of dimethylformamide, then add 25mmol of DIC, stir for 30min, then add 40mmol of N-hydroxysuccinimide, and avoid Stir the reaction for 16 hours, centrifuge to remove the precipitate, and the supernatant obtained by centrifugation is the reaction solution A; 2.5×10 -6 One mmol of keyhole limpet hemocyanin (KLH, molecular weight 8,000,000) was dissolved in 1.5 mL of 0.02 mol / L phosphate buffer solution (pH=7.4) to obtain solution B; solution B was added dropwise to reaction solution A, Stir overnight at 37°C, place the reaction solution in an ultrafiltration tube (molecular cutoff: 30 KD), centrifuge for 10 minutes, add 1 mL of 0.2mol / L PBS buffer solution with pH=7.4, centrifuge again, and repeat the operation 3 to 4 times , fully wash away the unreacted small molecular substances, collect the filtrate, take 1mL of the filtrate to pass through a 0.22μm filter membrane, and use high-performance li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com