Iridium complex containing hole transporting functional group, and electroluminescent device of iridium complex
An electroluminescent device, a technology of hole transport, which is applied in the direction of electroluminescent light sources, electric solid devices, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can solve the problem of insufficient thermal stability, Problems such as unsatisfactory energy level structure and triplet-triplet quenching can achieve the effects of improving electrical properties, increasing luminous efficiency, and reducing direct effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
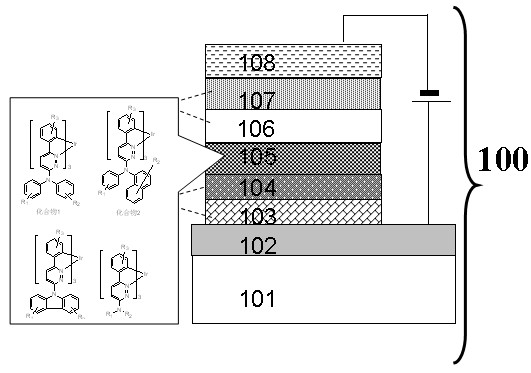
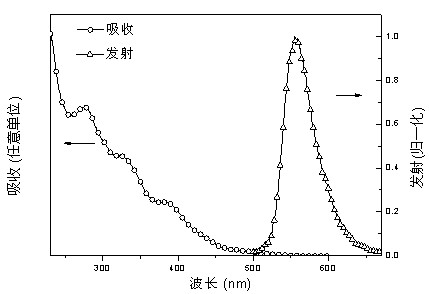
Image
Examples
Embodiment 1
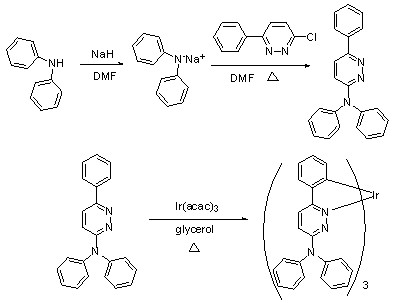
[0043] Example 1: Synthesis of N,N,6-triphenylpyridazin-3-amine, abbreviated as NPPya. Diphenylamine (2 mol) was dissolved in anhydrous DMF under nitrogen protection, and NaH (0.07 mol) was added to the solution in portions, and stirred at room temperature for 1 h; 3-chloro-6-phenylpyridazine ( 1.4 mol) was dissolved in DMF to dissolve, then the DMF solution of 3-chloro-6-phenylpyridazine was added to the above solution to react, heated to 50°C and stirred for 12 h, the whole process should be under the protection of nitrogen conduct. After the reaction was completed, water was added, accompanied by the formation of a pale yellow precipitate, which was filtered and dried to obtain a crude product. The crude product was purified by passing through a silica gel column to obtain a pure product.
Embodiment 2
[0044] Example 2: tris(N,N,6-triphenylpyridazin-3-amine)iridium(Ⅲ) , Abbreviated as the synthesis of IrNPPya. in N 2 Under protection, 206 mg (0.64 mmol) of NPPya and 80 mg (0.16 mmol) of iridium triacetylacetonate [Iridium(III) acetylacetonate] were added to 10 mL of glycerol, the temperature was raised to 50 °C, and air was pumped with a vacuum pump. Then the temperature was raised to 175 °C and refluxed for 12 h. The whole process was carried out under the protection of nitrogen. After a large amount of orange-yellow solid precipitated, stop the reaction, cool to room temperature, extract with dichloromethane, distill off the solvent under reduced pressure, wash with a small amount of methanol, and dry in vacuo to obtain an orange-yellow solid powder. Then it was separated by silica gel column chromatography using dichloromethane as the eluent to obtain an orange-yellow solid powder, which was a pure product. The NMR spectrum of IrNPPya is: 1 HNMR (400 MHz, TMS as int...
Embodiment 3
[0045] Example 3: Synthesis of [tris (9-(6-phenylpyridazin-3-yl)-9H-carbazole]iridium (III), referred to as IrCzPPya. in N 2 Under protection, add 126 mg (0.4 mmol) of CzPPya ligand and 48 mg (0.1 mmol) iridium triacetylacetonate [Iridium(III) acetylacetonate] into 6 mL glycerol [Glycerol], heat up to 50 °C, and use a vacuum pump Stir with air, then heat up to 185 °C, and reflux for 12 h. After a large amount of orange-yellow solid precipitated, stop the reaction, cool to room temperature, extract with dichloromethane, distill off the solvent under reduced pressure, wash with a small amount of methanol, and dry in vacuo to obtain an orange-yellow solid powder. Then it was separated by silica gel column chromatography using dichloromethane as the eluent to obtain an orange-yellow solid powder, which was a pure product. The NMR spectrum of IrCzPPya is: 1 H NMR (400MHz, TMS as internal standard, solvent DMSO-d 6 ) δ (ppm) = 8.76 (d, J = 9.2 Hz, 3H), 8.02 (d, J = 6.8 Hz, 3H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com