Novel levo-carnitine hydrogel patch and preparation method thereof
A technology of hydrogel patch and L-carnitine, applied in the field of medicine, can solve the problems of unsatisfactory skin penetration effect, high peeling strength, easy loss of viscosity, etc., so as to avoid peak-to-valley fluctuation and compatibility of blood drugs. Good, smooth absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
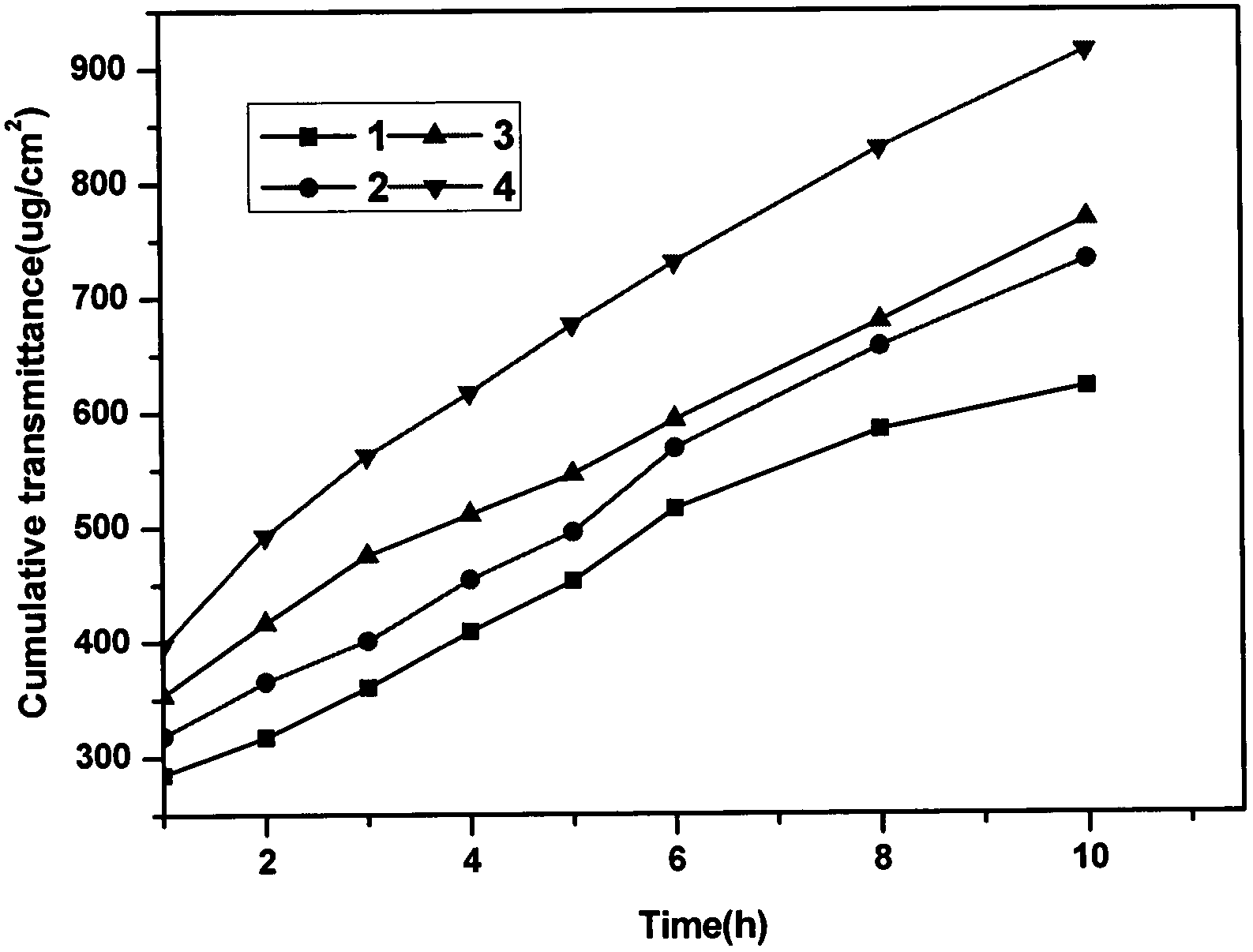
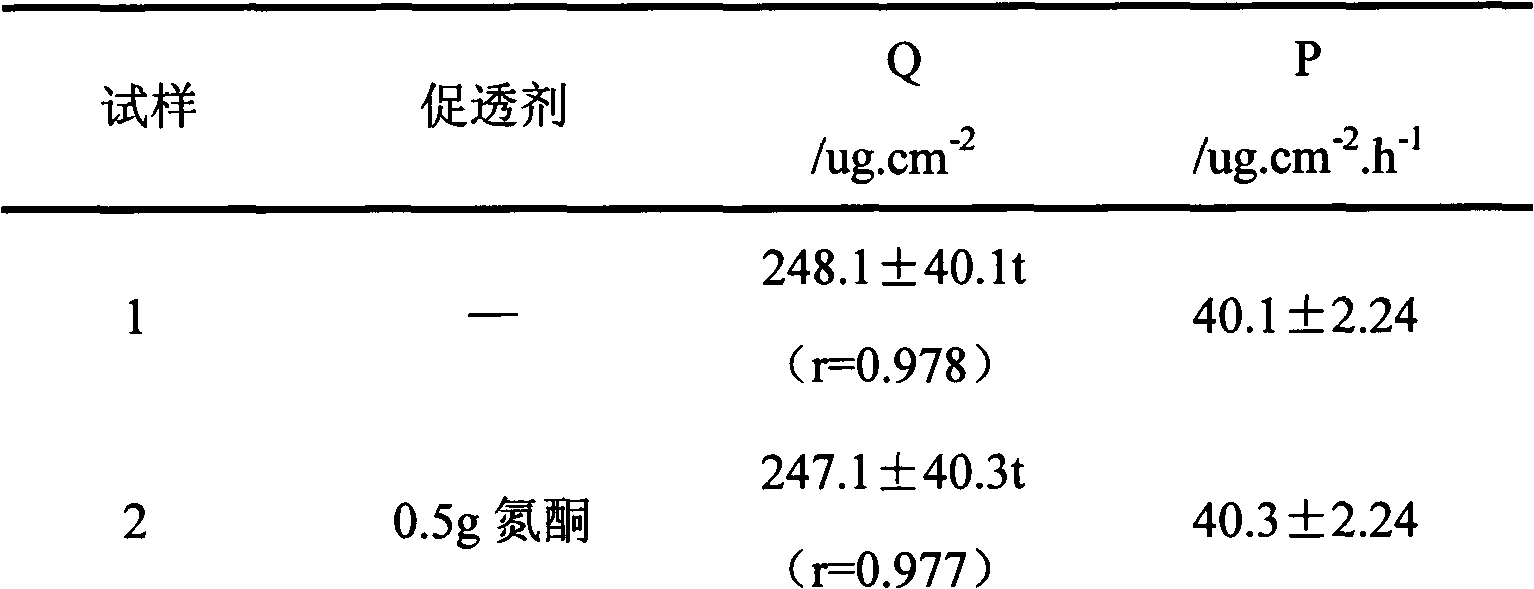
Image
Examples
Embodiment 1
[0033] 1. Take 6.00 g of sodium polyacrylate, 1.00 g of kaolin, 0.57 g of glyoxate, and 0.40 g of EDTA, and disperse them in a mixture of 35.00 g of glycerin and propylene glycol, and stir at a constant speed for 30 minutes at room temperature to obtain a uniform dispersed phase I.
[0034] 2. Take 1.00 g of sodium alginate, 5.00 g of L-carnitine, and 1.50 g of tartaric acid, and dissolve them in 60.00 g of distilled water. Stir at a constant speed at 90°C for 30 minutes to obtain a solution phase II.
[0035] 3. Mix the uniformly dispersed phase I with the solution phase II, and stir at a constant speed for 30 minutes until the mixture is uniform. Dry at 75° C. for 15 minutes to obtain a drug-containing matrix.
[0036] 4. Evenly coat the drug-containing matrix on the non-woven fabric of the backing layer, cover with a protective layer, mold it, and then cut it into the required sample size as needed.
Embodiment 2
[0038] 1. Take 6.00g of sodium polyacrylate, 1.00g of kaolin, 0.57g of aluminum glyoxate, 0.40g of EDTA, and 0.5g of vitamin E, and disperse them in a mixture of 35.00g of glycerin and propylene glycol, and stir at a constant speed for 30 minutes at room temperature to obtain a uniform dispersion Phase I.
[0039] 2. Dissolve 1.00g of sodium alginate, 5.00g of L-carnitine and 1.50g of tartaric acid in 60.00g of distilled water. Stir at a constant speed at 90°C for 30 minutes to obtain a solution phase II.
[0040] 3. Mix the uniformly dispersed phase I with the solution phase II, and stir at a constant speed for 30 minutes until the mixture is uniform. Dry at 75° C. for 15 minutes to obtain a drug-containing matrix.
[0041] 4. Evenly coat the drug-containing matrix on the non-woven fabric of the backing layer, cover with a protective layer, mold it, and then cut it into the required sample size as needed.
Embodiment 3
[0043] 1. Take 6.00 g of sodium polyacrylate, 1.00 g of kaolin, 0.57 g of glyoxate, and 0.40 g of EDTA, and disperse them in a mixture of 35.00 g of glycerin and propylene glycol, and stir at a constant speed for 30 minutes at room temperature to obtain a uniform dispersed phase I.
[0044] 2. Dissolve 1.00g of sodium alginate, 5.00g of L-carnitine, 1.50g of tartaric acid and 0.5g of menthol in 60.00g of distilled water. Stir at a constant speed at 90°C for 30 minutes to obtain a solution phase II.
[0045] 3. Mix the uniformly dispersed phase I with the solution phase II, and stir at a constant speed for 30 minutes until the mixture is uniform. Dry at 75° C. for 15 minutes to obtain a drug-containing matrix.
[0046] 4. Evenly coat the drug-containing matrix on the non-woven fabric of the backing layer, cover with a protective layer, mold it, and then cut it into the required sample size as needed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com