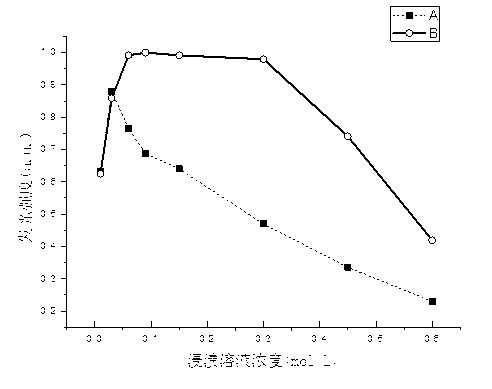
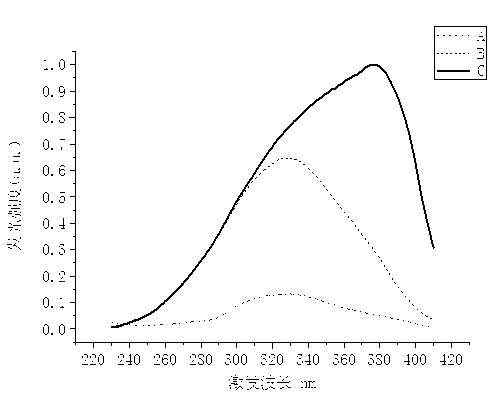
Preparation method of near ultraviolet-excited high silica blue-light-emitting glass
A high-silicon, near-ultraviolet technology, applied in the field of high-silicon blue luminescent glass, can solve the problem of limited doping concentration of porous glass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take analytically pure chemical reagents, according to SiO 2 38.78wt%, Na 2 CO 3 9.79wt%, H 3 BO 3 42.42wt%, Al(OH) 3 3.73wt%, CaCO 3 5.01wt%, CuCl 2 The ratio of 0.27wt% is used to prepare mixed raw materials. After mixing and grinding evenly, put it into a platinum crucible, and after melting at a high temperature of 1450°C for 40 minutes, cool it on an iron plate at 400°C to form borosilicate glass.
[0027]Put the borosilicate glass into a high-temperature furnace and heat-treat at 670° C. for 20 hours, and then cut it into glass pieces of 5 mm×5 mm×1 mm. Put the glass sheet into a sealed autoclave, and place it at 98°C for five times of acid treatment for 24 hours each time; the first time immerse it in 1mol / L nitric acid solution at the ratio of 50ml acid solution / gram of glass, and the second time Immerse in 1mol / L nitric acid solution at the ratio of 10ml acid solution / gram glass for the second time, immerse in 0.3mol / L nitric acid solution at the ...
Embodiment 2
[0032] Take analytically pure chemical reagents, according to SiO 2 30.91wt%, Na 2 CO 3 7.80wt%, H 3 BO 3 54.10wt%, Al(OH) 3 2.97wt%, CaCO 3 4.00wt%, Cr 2 o 3 The ratio of 0.22wt% is used to prepare mixed raw materials. After mixing and grinding evenly, put it into a corundum crucible, and after melting at 1400°C for 45 minutes, cool it on an iron plate at 100°C to form borosilicate glass.
[0033] Put the borosilicate glass into a high-temperature furnace and heat-treat at 650° C. for 10 hours, and then cut it into glass pieces of 5 mm×5 mm×1 mm. Put the glass into a sealed autoclave and place it at 90°C for five times of acid treatment for 12 hours each time; the first time immerse it in 1mol / L hydrochloric acid solution at a ratio of 50ml per g of glass, and the second to Immerse in 1mol / L hydrochloric acid solution at a ratio of 10ml per g glass for five times. Before changing the acid solution, place the glass at 200°C for 5 hours to promote the precipitat...
Embodiment 3
[0036] Take analytically pure chemical reagents, according to SiO 2 20.47wt%, Na 2 CO 3 3.27wt%, H 3 BO 3 46.05wt%, Al(OH) 3 2.92wt%, the ratio of waste bottle glass 27.29wt% is used to prepare mixed raw materials. After mixing and grinding evenly, put it into a platinum crucible, and after melting at 1500°C for 30 minutes, cool it on an iron plate at 300°C to form borosilicate glass.
[0037] Put the borosilicate glass into a high-temperature furnace and heat-treat at 590° C. for 80 hours, and then cut it into glass pieces of 5 mm×5 mm×1 mm. Put the glass in a sealed autoclave and place it at 100°C for four times of acid treatment for 48 hours each time; the first time immerse it in 1mol / L nitric acid solution at the ratio of 50ml acid solution / gram of glass, and the second time Immerse in 1mol / L nitric acid solution at the ratio of 10ml acid solution / gram glass for the first time, and immerse in 0.3mol / L nitric acid solution at the ratio of 10ml acid solution / gram ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com