pH-responsive 6-arm star block copolymer and preparation method and application thereof
A technology of block copolymers and polymers, which can be used in pharmaceutical combinations, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., and can solve the problems of insufficient stability and controlled release performance of multi-arm star block copolymers. , to achieve the effect of improving pH response sensitivity and release efficiency, controlling drug release, and improving controlled release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
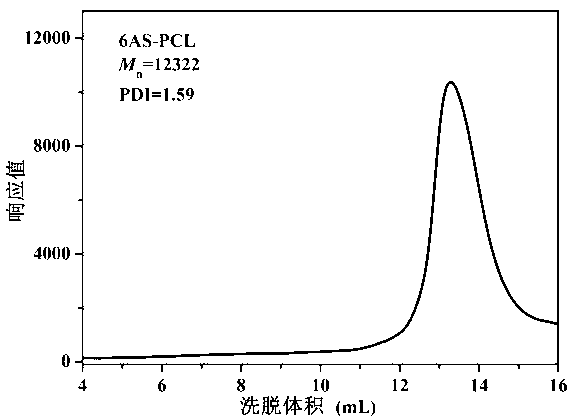
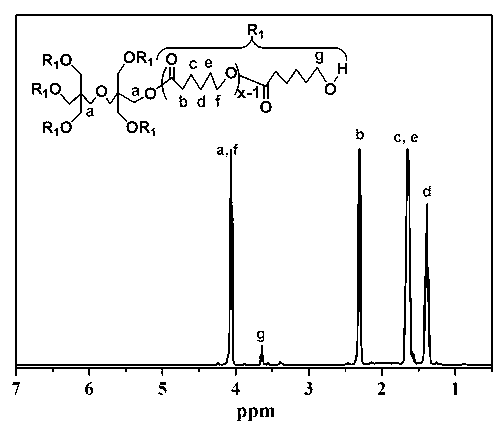
[0068] (1) Synthesis of polymers grafted with hydrophobic groups: Put a stirrer and 0.254g of pentaerythritol in a reaction bottle, seal it and pump it in a vacuum-argon three times, then add 12g of monomer e-CL and 0.012g of Sn (October) 2 , after three cycles of freezing-pumping-heating with liquid nitrogen, stir the reaction in an oil bath at 120°C under the protection of argon for 36h, after the completion of the reaction, cool to room temperature, evaporate toluene under reduced pressure, add 50mL THF to dilute, and then use 300mL methanol / water (1:1 volume ratio) at 0°C was precipitated and dried in vacuum at 45°C for 24 hours to obtain a white powder, which was the polymer 6AS-PCL grafted with hydrophobic groups, with a yield of 93%. M n =12322, PDI=1.59;
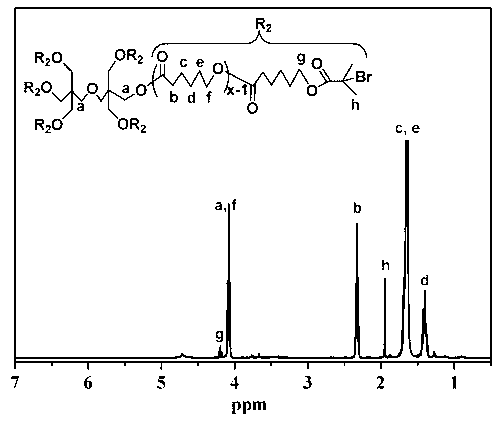
[0069] (2) Synthesis of macromolecular initiators: take a baked and dried 250mL three-neck flask, add 12g 6AS-PCl and 150mL THF, seal it and pass argon for 10min, inject 3.04g TEA after sealing, cool to 0°C with an...
Embodiment 2
[0080] (1) Synthesis of 6AS-PCL: Put a stirrer and 0.254g of pentaerythritol in the reaction bottle, seal it and pump it to a vacuum-argon three times, then use a syringe to inject 10g of monomer e-CL and 0.020g of Sn(Oct) 2 Add it into the reaction flask, use liquid nitrogen for three times of freezing-pumping-heating cycle, and stir the reaction in an oil bath at 140°C under the protection of argon for 24 hours. After the reaction is completed, cool to room temperature, evaporate toluene under reduced pressure, and add 50mL THF Diluted, then precipitated with 300mL 0°C methanol / water (1:1 volume ratio), and dried in vacuum at 45°C for 24h to obtain a white powder, 6AS-PCL, with a yield of 86%. M n =10918, PDI=1.62;
[0081] (2) Synthesis of 6AS-PCL-Br: Take a baked and dried 250mL three-neck flask, add 10g 6AS-PCl and 150mL THF, seal it and pass argon for 10min, inject 2.43g TEA after sealing, cool to 0°C with an ice-water bath, Then add 5.52g of 2-bromoisobutyryl bromide,...
Embodiment 3
[0084] (1) Synthesis of 6AS-PCL: Put a stirrer and 0.254g of pentaerythritol in the reaction bottle, seal it and pump the vacuum-argon gas three times, then inject 16g of monomer e-CL and 0.008g of Sn(Oct) 2 Add it into the reaction flask, use liquid nitrogen for three times of freezing-pumping-heating cycle, and stir the reaction in an oil bath at 110°C under the protection of argon for 48h. After the reaction is completed, cool to room temperature, evaporate the toluene under reduced pressure, and add 50mL THF Diluted, then precipitated with 300mL 0°C methanol / water (1:1 volume ratio), and dried in vacuum at 45°C for 24h to obtain a white powder, 6AS-PCL, with a yield of 89%. M n =17543, PDI=1.60;
[0085] (2) Synthesis of 6AS-PCL-Br: Take a baked and dried 250mL three-neck flask, add 16g 6AS-PCl and 150mL THF, seal it and pass argon for 10min, inject 3.64g TEA after sealing, cool to 0°C with an ice-water bath, Then add 8.28g of 2-bromoisobutyryl bromide, react at 0°C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Critical micelle concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com