Real-time fluorescence nucleic acid constant temperature amplification detection kit of general influenza a virus (IAV)
A type A influenza virus, real-time fluorescence technology, applied in the field of probes and related kits, primers, can solve the problems of long detection cycle, amplification product contamination, low sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
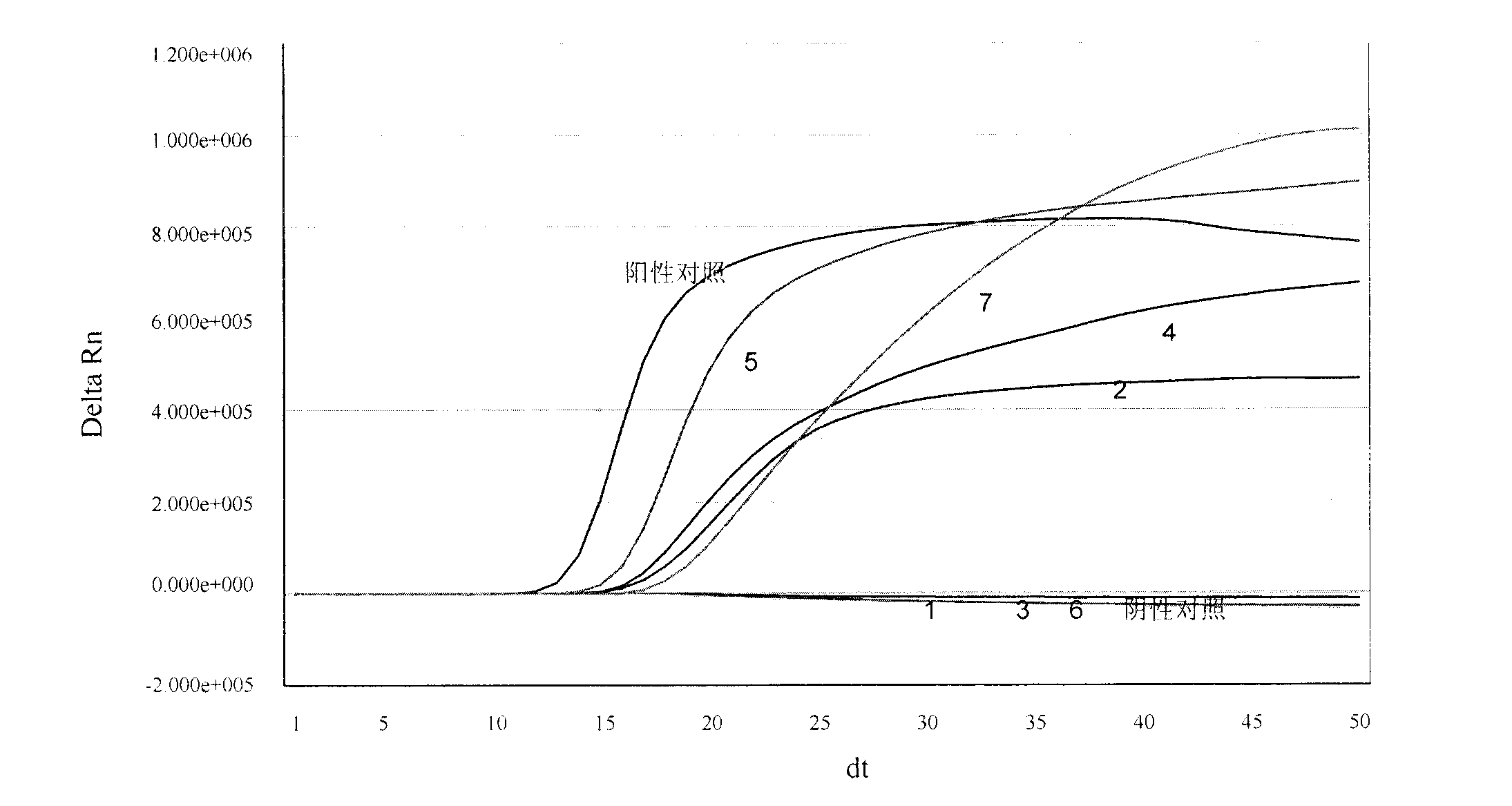
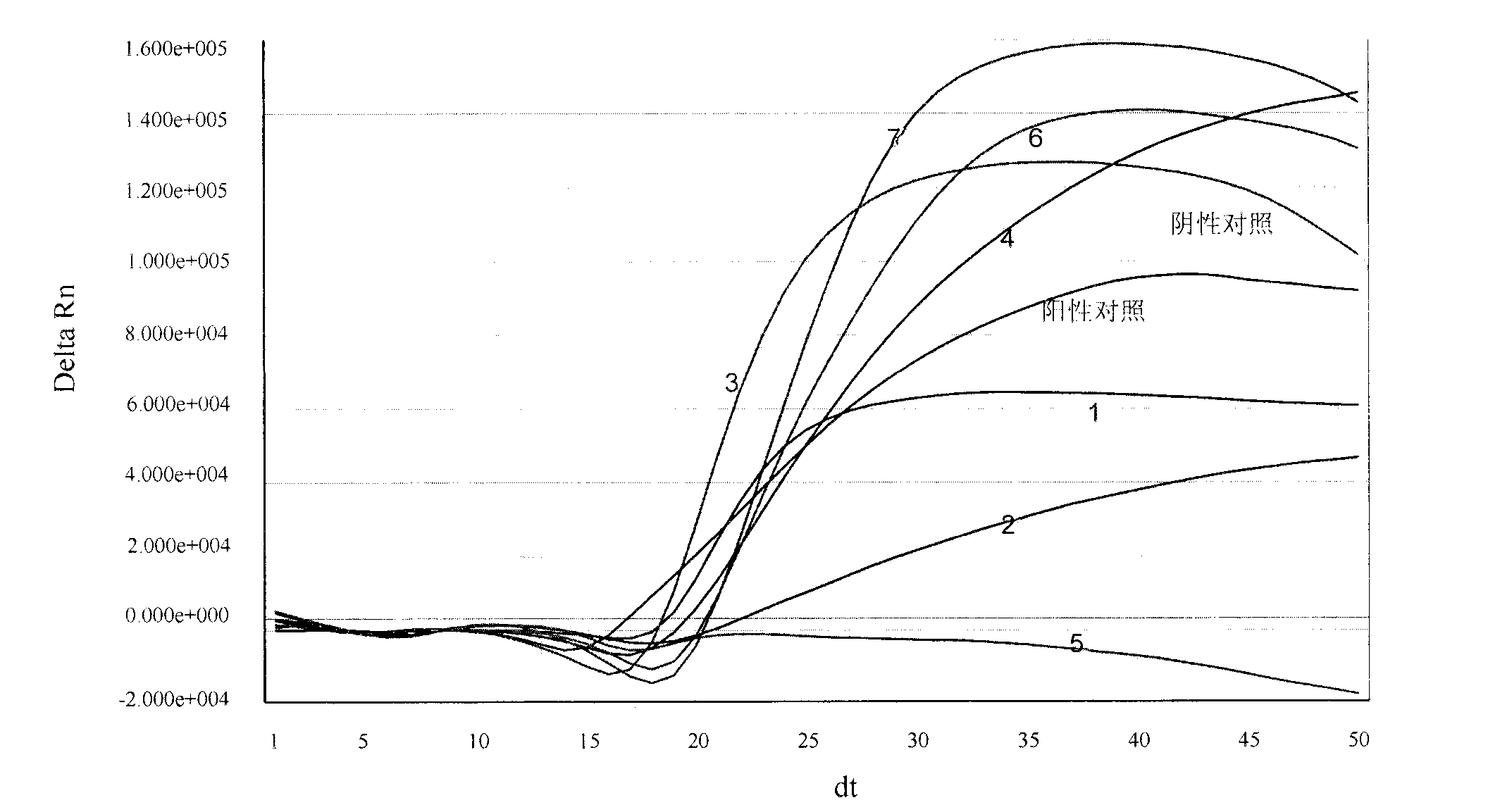
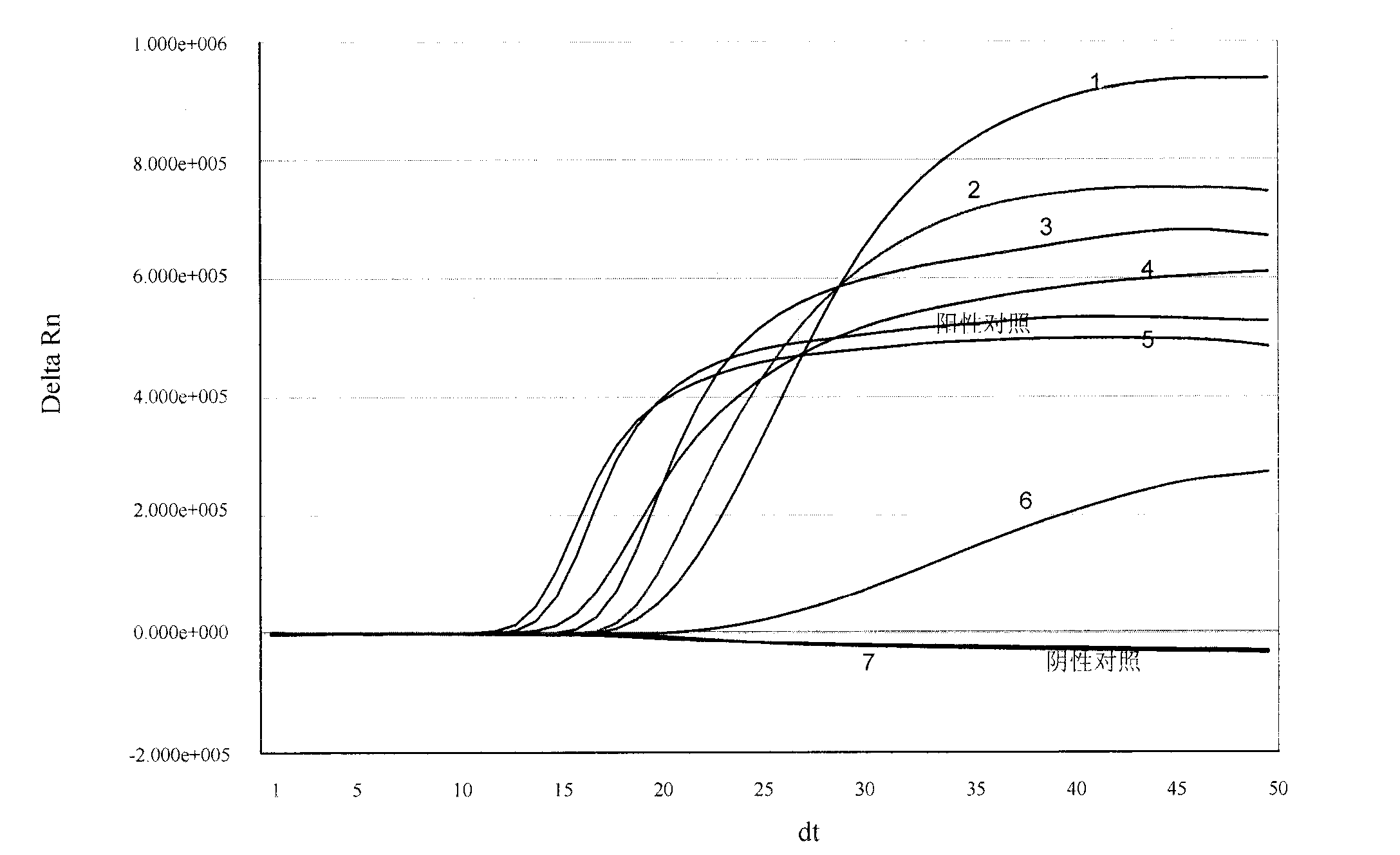
[0100] Embodiment 1, be used for the design of the special primer of real-time fluorescent nucleic acid constant temperature amplification detection universal type influenza A virus (IAV) and probe
[0101] The present invention selects no secondary structure and a highly conserved segment in the IAV virus M gene as the amplified target sequence region (its nucleotide sequence is shown in sequence 1 in the sequence table), and according to the principle of primer probe design, DNA ATAR, DNAman software and artificial design are used for real-time fluorescent nucleic acid constant temperature amplification to detect the special-purpose primer and probe sequence of universal influenza A virus (IAV), obtain following specific sequence:
[0102] (1) a capture probe (TCO, Target Capture Oligo) that can be specifically combined with the target nucleic acid (IAV RNA) sequence of the general type influenza A virus (IAV) shown in sequence 1 in the sequence listing (TCO, Target Capture O...
Embodiment 2
[0106] Embodiment 2, prepare the real-time fluorescent nucleic acid constant temperature amplification detection kit of universal influenza A virus (IAV)
[0107]Using the special primers and probes provided in Example 1, a real-time fluorescent nucleic acid constant temperature amplification detection kit for general-purpose influenza A virus (IAV) of the present invention was obtained. The kit includes capture probe (TCO, Target Capture Oligo), T7 primer, nT7 primer, IAV detection probe, internal standard detection probe, M-MLV reverse transcriptase and T7 RNA polymerase.
[0108] The capture probe exists in the nucleic acid extraction solution, the T7 primer, nT7 primer, IAV detection probe, and internal standard detection probe exist in the IAV detection solution, and the M-MLV reverse transcriptase and T7 RNA polymerase The enzyme exists in the SAT enzyme solution. Specifically, the kit is divided into A box (specimen processing unit) stored at 2-30°C and B box (nucleic a...
Embodiment 3
[0141] Example 3. Real-time fluorescent nucleic acid constant temperature amplification detection of clinical sample throat swab
[0142] Use the kit of the present invention (see embodiment 2 for composition) to detect the general type influenza A virus (IAV) in the throat swab of clinical sample, and specific method comprises the following steps:
[0143] (1) Sample collection, transportation and storage
[0144] The clinician will collect the specimens according to the actual situation. The test specimens are throat swabs. The collection method is: wipe the posterior pharyngeal wall and the pharyngeal tonsils on both sides with a special sampling cotton swab, invade the swab into 3-5mL normal saline, and send it sealed for inspection. Within 48 hours after the sample is collected, store it at 4°C and send it to the Influenza Surveillance Network Laboratory (or store it at -70°C for testing, and deliver it within one week).
[0145] (2) Nucleic acid extraction
[0146] 2.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com