Method for preparing nanometer platinum/titanium dioxide nanotube electrode through cyclic voltammetry electrodeposition
A cyclic voltammetry and titanium dioxide technology, which is applied in the field of photoelectric catalysis, can solve the problems of low current density, uneven deposition layer, and high recombination rate of photogenerated carriers, and achieves fine Pt nanoparticles, excellent photoelectric catalytic performance, and wide application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
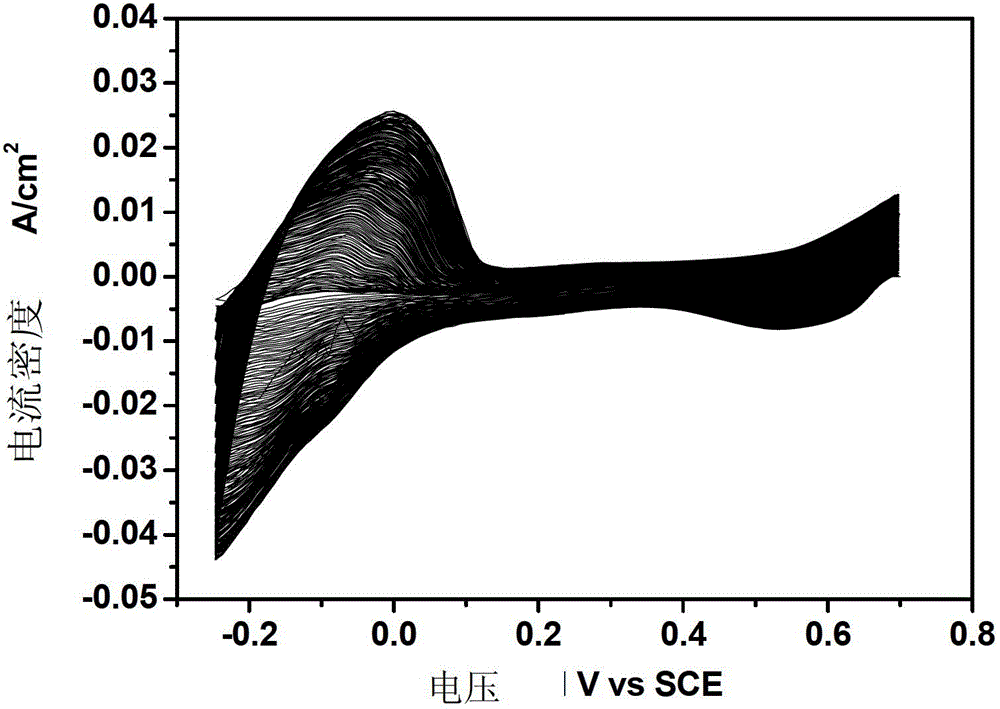
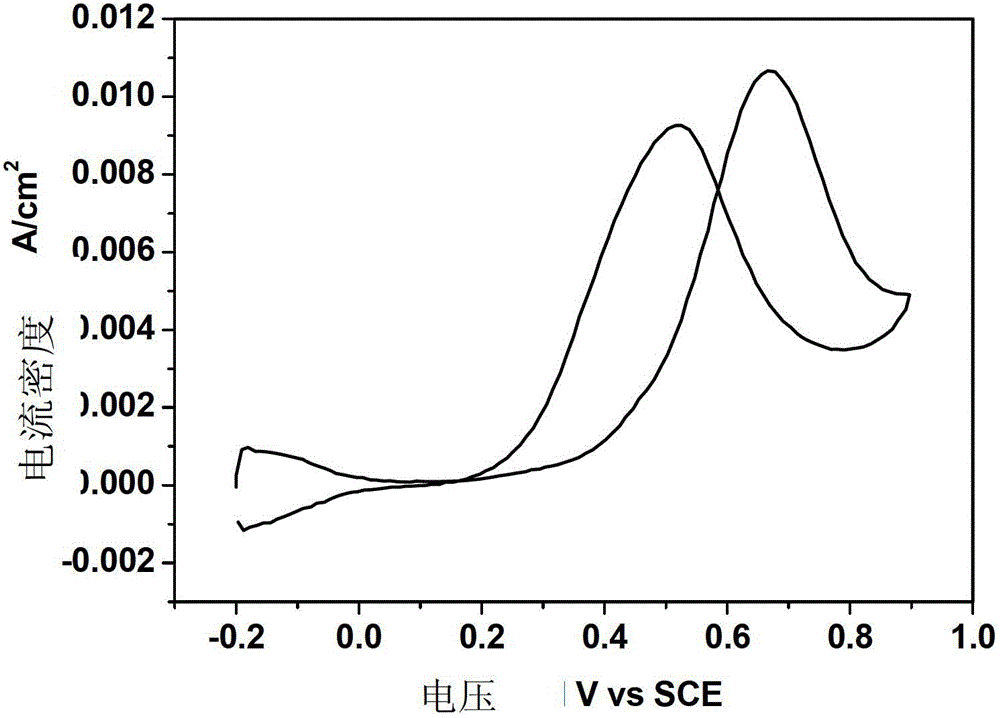
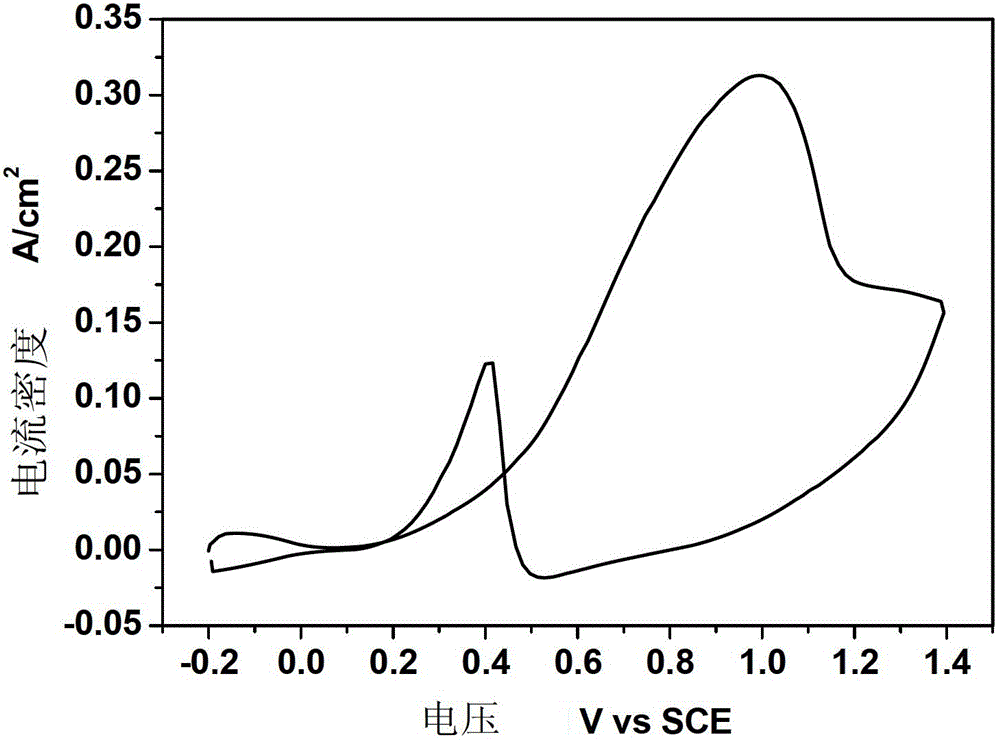
[0022] Example 1. After surface treatment of 1.0mm*1.0mm TA1 titanium sheet, put 1g / LNH 4 HF 2 , 50g / LH 2 The ethylene glycol solution of O was subjected to anodic oxidation at a constant voltage of 10V and a temperature of 5°C for 1h, with constant mechanical stirring during the period. Prepared TiO 2 After the nanotubes are rinsed, they are placed in an electrolyte solution with a composition of 1.0mM chloroplatinic acid + 0.5M sulfuric acid. The scanning speed is 10mV / s, the voltage scanning range is -1 to 0.3V, and the scanning cycle is 20 times. . The test of the prepared electrode to catalyze methanol is: put the electrode into 0.5M H 2 SO 4 +0.5MCH 3 In the solution of OH, the reference electrode is a saturated calomel electrode, the counter electrode is a platinum electrode, and the working electrode is Pt-TiO 2 / Ti nanotube electrodes. Nitrogen gas was introduced into the solution before the measurement to remove the oxygen dissolved in the solution, and the m...
example 2
[0023] Example 2. Put 5g / L NH 4 HF 2 , 200g / LH 2 The ethylene glycol solution of O was subjected to anodic oxidation at a constant voltage of 60V and a temperature of 30°C for 6h, with constant mechanical stirring during the period. Prepared TiO 2 After the nanotubes were rinsed, they were placed in an electrolyte solution with a composition of 10.0mM chloroplatinic acid + 0.5M sulfuric acid. The parameters of cyclic voltammetry electrodeposition platinum were: scanning speed was 100mV / s, voltage scanning range was -0.25~0.7, scanning The cycle is 200 times. The test of the prepared electrode to catalyze methanol is: put the electrode into 0.5M H 2 SO 4 +0.5M CH 3 In the solution of OH, the reference electrode is a saturated calomel electrode, the counter electrode is a platinum electrode, and the working electrode is Pt-TiO 2 / Ti nanotube electrodes. Nitrogen gas was introduced into the solution before the measurement to remove the oxygen dissolved in the solution, an...
example 3
[0024] Example 3. Put 3g / L NH 4 HF 2 , 100g / LH 2 The ethylene glycol solution of O was subjected to anodic oxidation at a constant voltage of 30V and a temperature of 10°C for 2h, with constant mechanical stirring during the period. Prepared TiO 2 After the nanotubes were rinsed, they were placed in an electrolyte solution with a composition of 4.0mM chloroplatinic acid + 0.5M sulfuric acid. The parameters of cyclic voltammetry electrodeposition platinum were: scanning speed was 50mV / s, voltage scanning range was -0.5 to 1.0, scanning The cycle is 50 times. The test of the prepared electrode to catalyze methanol is: put the electrode into 0.5M H 2 SO 4 +0.5M CH 3 In the solution of OH, the reference electrode is a saturated calomel electrode, the counter electrode is a platinum electrode, and the working electrode is Pt-TiO 2 nanotube electrodes. Nitrogen gas was introduced into the solution before the measurement to remove the oxygen dissolved in the solution, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com