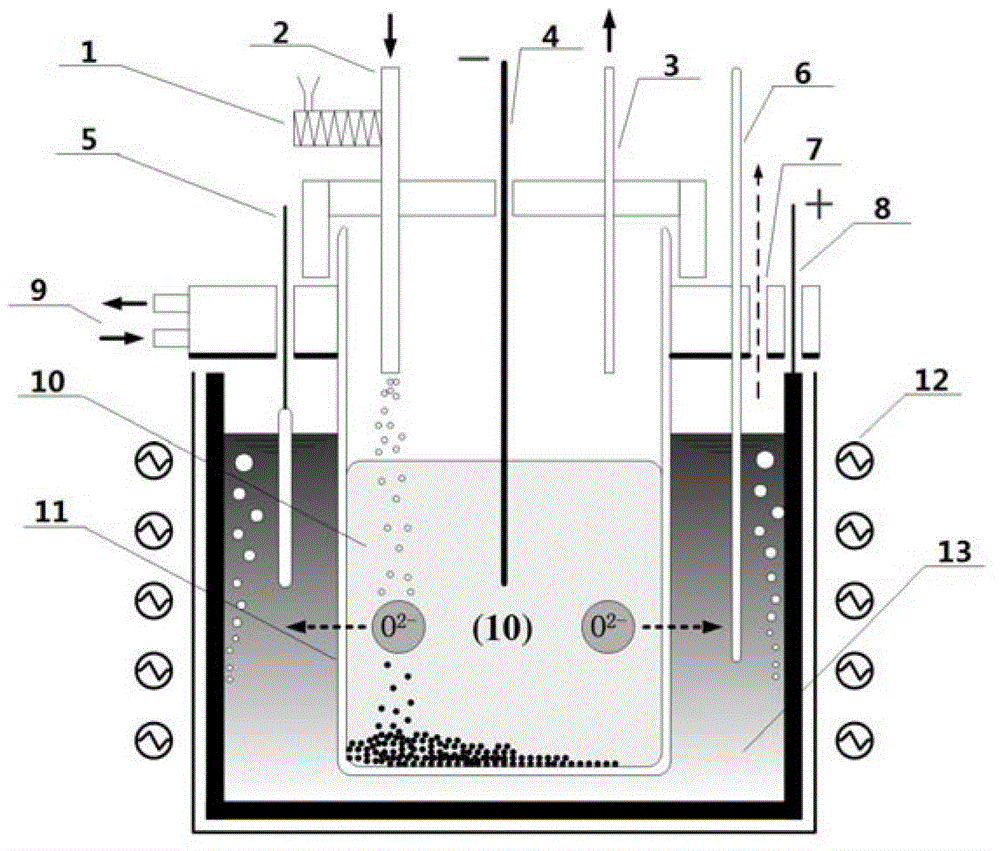
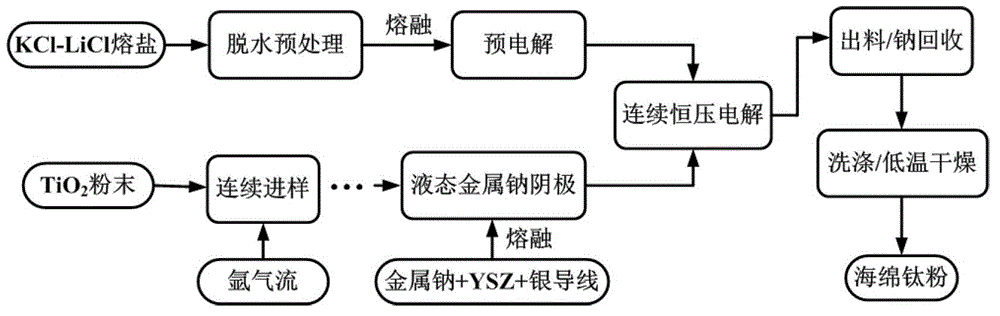
Method and electrolytic tank for producing metal titanium through directive electrolysis of titanium dioxide
A technology of titanium dioxide and electrolytic cells, applied in the field of electrolytic cells, can solve the problems that the reducing agent Ca regeneration ability is not enough to support a long-term, continuous reduction process, slow solid phase migration process, slow electrolysis rate, etc., to achieve continuous The production process, simplify the reduction process route, increase the effect of corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The KCl-LiCl molten salt with a molar ratio of 47.0:53.0 was dehydrated at 300°C for 12 hours and transferred to a graphite crucible while it was still hot, and the temperature was rapidly raised to 450°C in the reactor for melting (the upper cover was filled with circulating cooling water ), the molten salt depth is controlled at 1 / 2 to 2 / 3 of the height of the reactor, and the time required for the heating and melting process does not exceed 30 minutes. The pre-electrolysis process was carried out at 2.0V and 0.5h with the iron-chromium-aluminum high-temperature-resistant conductor as the pre-electrolysis cathode and the graphite crucible as the anode. Introduce 600ml / min high-purity argon gas into the YSZ tube for 0.5h, then add 300g solid metal sodium, seal the upper cover and YSZ tube, install the cathode wire and continue to inject high-purity argon gas. Connect the above-mentioned combined cathode to the upper cover of the electrolytic cell and slowly lower the c...
Embodiment 2
[0029] A certain amount of KCl-LiCl molten salt with a molar ratio of 40.8:59.2 was dehydrated at 300°C for 12 hours and transferred to a graphite crucible while it was still hot, and the temperature was rapidly raised to 450°C in the reactor for melting (the upper cover was circulated Cooling water), the depth of molten salt is controlled at 1 / 2 to 2 / 3 of the height of the reactor, and the time required for the heating and melting process does not exceed 30 minutes. The pre-electrolysis process was carried out at 2.0V and 0.5h with the iron-chromium-aluminum high-temperature-resistant conductor as the pre-electrolysis cathode and the graphite crucible as the anode. Introduce 600ml / min high-purity argon gas into the YSZ tube for 0.5h, then add 300g solid metal sodium, seal the upper cover and YSZ tube, install the cathode wire and continue to inject high-purity argon gas. Connect the above-mentioned combined cathode to the upper cover of the electrolytic cell and slowly lower ...
Embodiment 3
[0032] A certain amount of KCl-LiCl molten salt with a molar ratio of 40.8:59.2 was dehydrated at 300°C for 12 hours and transferred to a graphite crucible while it was still hot, and the temperature was rapidly raised to 450°C in the reactor for melting (the upper cover was circulated Cooling water), the depth of molten salt is controlled at 1 / 2 to 2 / 3 of the height of the reactor, and the time required for the heating and melting process does not exceed 30 minutes. The pre-electrolysis process was carried out at 2.0V and 0.5h with the iron-chromium-aluminum high-temperature-resistant conductor as the pre-electrolysis cathode and the graphite crucible as the anode. Introduce 600ml / min high-purity argon gas into the YSZ tube for 0.5h, then add 400g solid metal sodium, seal the upper cover and YSZ tube, install the cathode wire and continue to inject high-purity argon gas. Connect the above-mentioned combined cathode to the upper cover of the electrolytic cell and slowly lower ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com