Resin ointment with high drug release, and preparation method and application thereof
A resin type and ointment technology, which is used in pharmaceutical formulations, medical preparations with non-active ingredients, and ointment transportation, can solve the problems of strong adhesion and poor drug release performance of resin type ointments, and achieve good drug release and stickiness. The effect of moderate patch performance and high drug release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Preparation of resin paste
[0049]
[0050] With the above quality raw materials, proceed as follows:
[0051] 1. Add the thermoplastic elastomer SIS, plasticizer, and 50% antioxidant into the reactor, blow nitrogen for 5 minutes to remove the air, heat up until the mixture melts, and at the same time pass nitrogen protection, control the temperature between 120℃ and 150℃, stir , Until the mixture forms a uniform and transparent viscous colloid.
[0052] 2. Put the tackifying resin and the remaining 50% of the antioxidant into the above mixture, control the temperature between 100°C and 110°C, stir for half an hour, while protecting with nitrogen, until the mixture forms a uniform and transparent viscoelastic gel.
[0053] 3. Cool the above colloid to below 100°C and pour it out.
[0054] 2. Preparation of joint pain relief cream
[0055] 1. Prepare the traditional Chinese medicine resin ointment by the above method in Example 1;
[0056] 2. Weigh 100g of resin paste matrix, a...
Embodiment 2
[0095] 1. Preparation of resin paste
[0096]
[0097] Prepare according to the above composition ratio, and the method is the same as in Example 1.
[0098] 2. Preparation of Joint Pain Relieving Ointment: Repeat the method of Example 1 except that the resin paste base prepared in Example 2 is used to replace the base prepared in Example 1.
[0099] 3. Product testing:
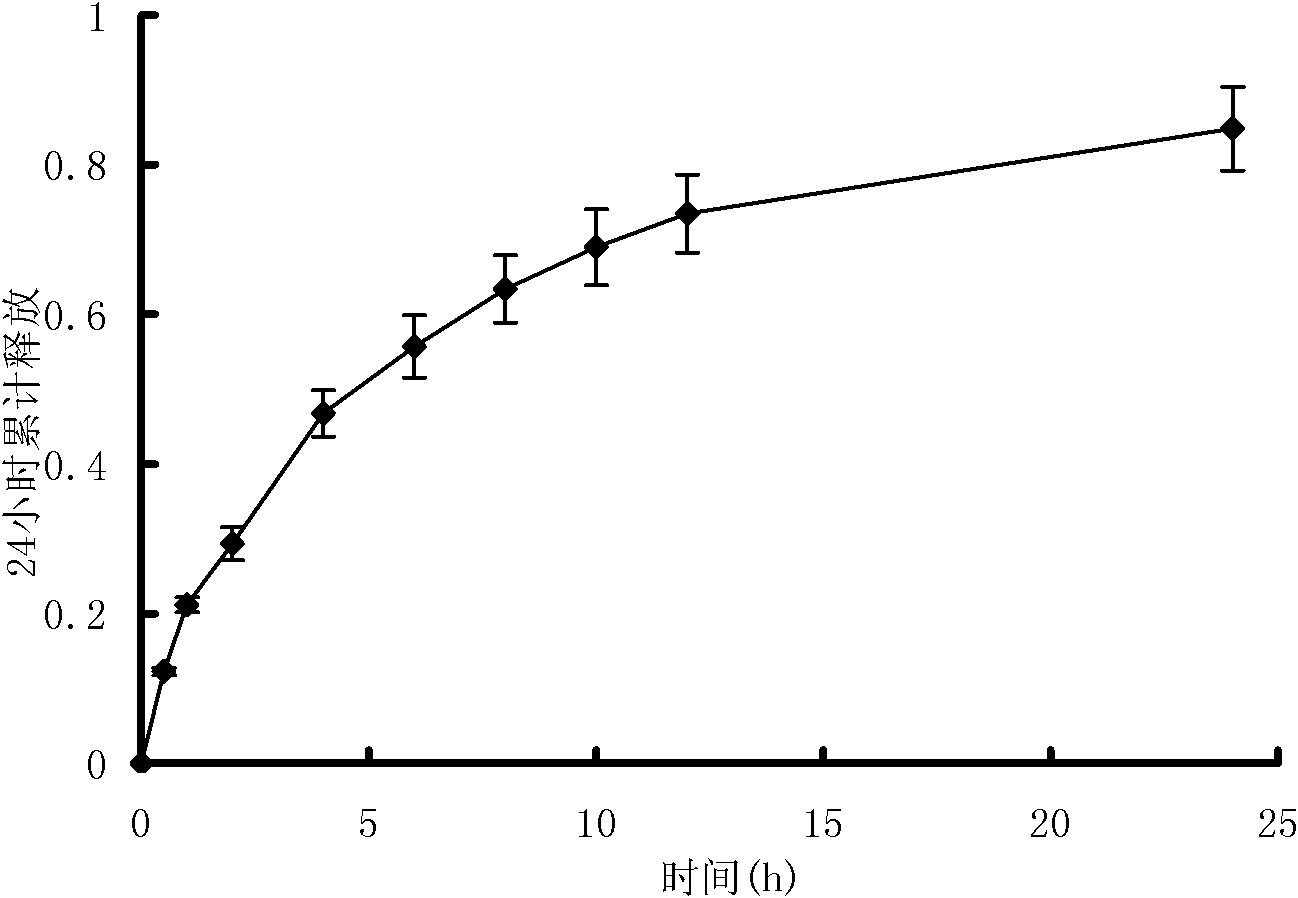
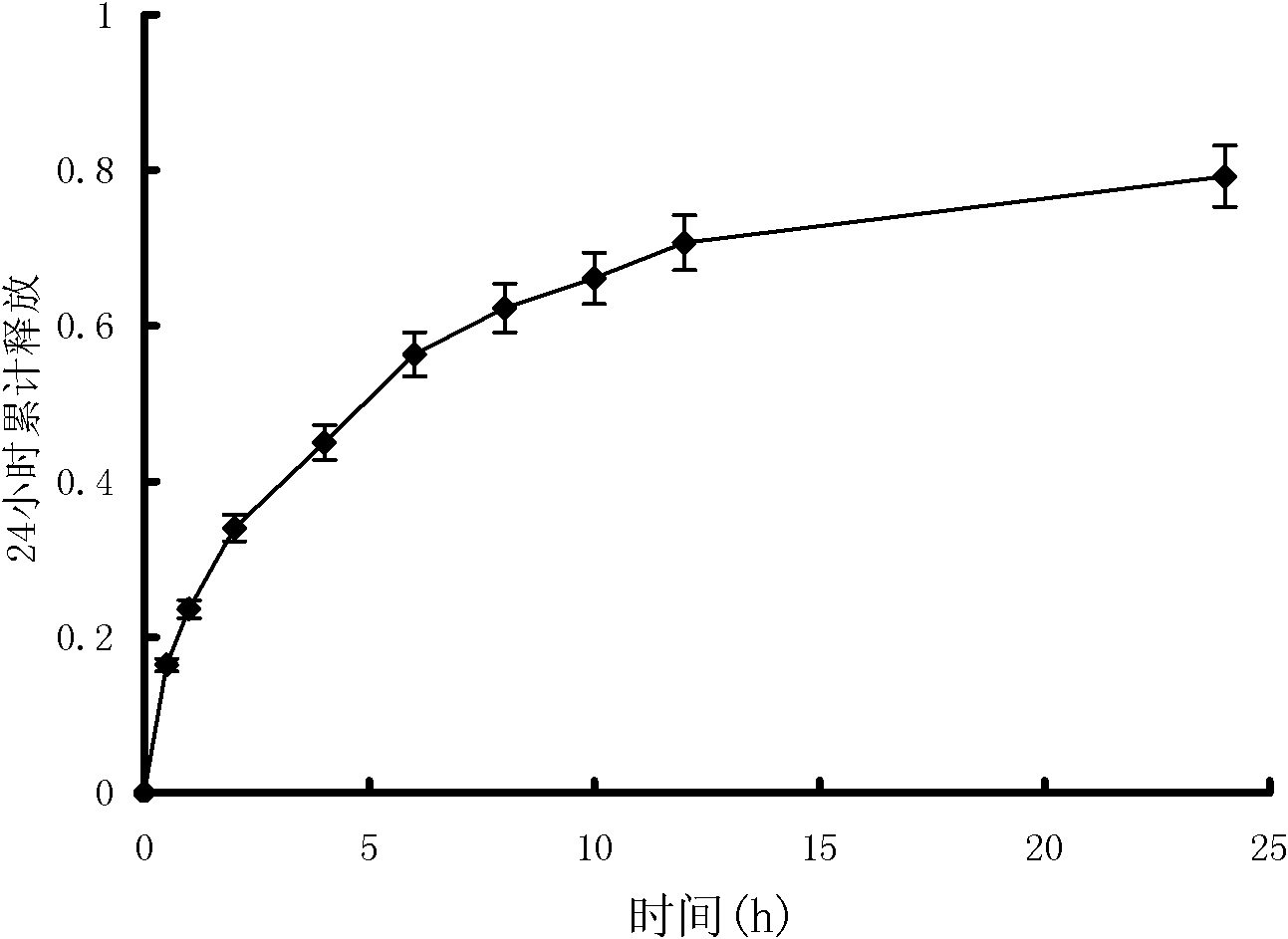
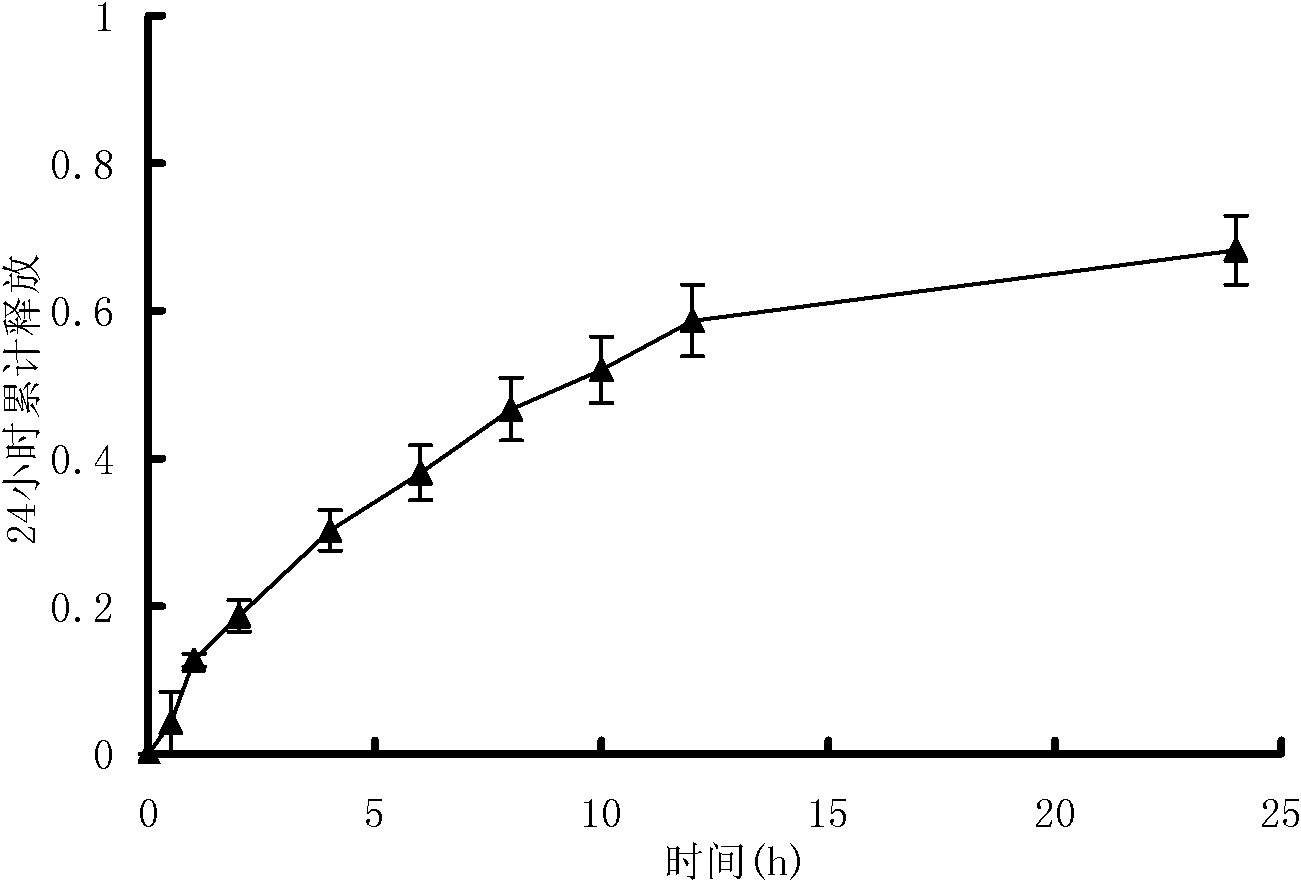
[0100] The joint pain relief ointment prepared in Example 2 was investigated for initial viscosity, stickiness, peel strength, drug loading, and drug release performance. The method is the same as in Example 1. The test results are shown in Tables 4, 5, 6 and image 3 , Figure 4 .
[0101] Table 4
[0102]
[0103] The results in Table 4 show that the plaster prepared in Example 2 has moderate adhesion, peel strength between 1N and 2N, and good air permeability.
[0104] Table 5 Effects on the release of camphor in patches
[0105]
[0106] Table 5 and image 3 The results show that the plaster prepared in Example 2 ha...
Embodiment 3
[0111] 1. Preparation of resin paste:
[0112]
[0113] With the above quality raw materials, proceed as follows:
[0114] 1. Add the thermoplastic elastomer SIS, plasticizer, and 50% antioxidant into the reactor, blow nitrogen for 5 minutes to remove the air, heat up until the mixture melts, and at the same time pass nitrogen protection, control the temperature between 120℃ and 150℃, stir , Until the mixture forms a uniform and transparent viscous colloid.
[0115] 2. Put the tackifying resin and 50% antioxidant into the above mixture, control the temperature between 100°C and 110°C, stir for half an hour while protecting with nitrogen until the mixture forms a uniform and transparent viscoelastic gel; then add it Filler and organic acid, stir to make the dispersion uniform;
[0116] 3. Cool the above colloid to below 100°C and pour it out.
[0117] 2. Preparation of joint pain relief cream
[0118] 1. Prepare the traditional Chinese medicine resin ointment by the above method of Exam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com