Preparation method of 1*1-type manganese oxide octahedral molecular sieves
A technology of pyrolusite and permanganate, applied in molecular sieve compounds, molecular sieves and alkali exchange compounds, chemical instruments and methods, etc., can solve problems such as unfavorable practical application, high energy consumption, etc. The effect of high secondary yield and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: adopt MnSO 4 As the divalent manganese source, take KMnO 4 Preparation of pyrolusite (β-MnO 2 ).
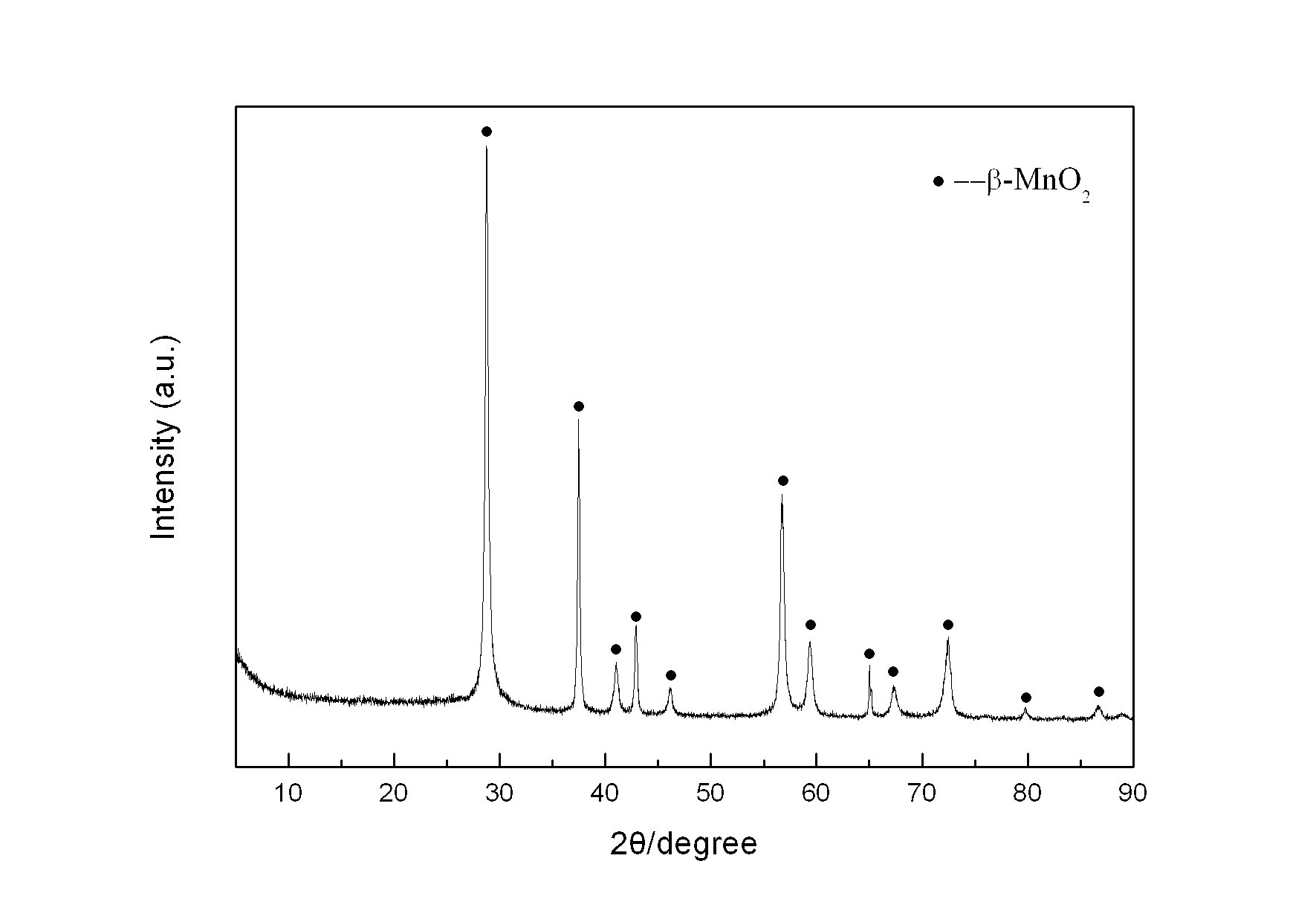
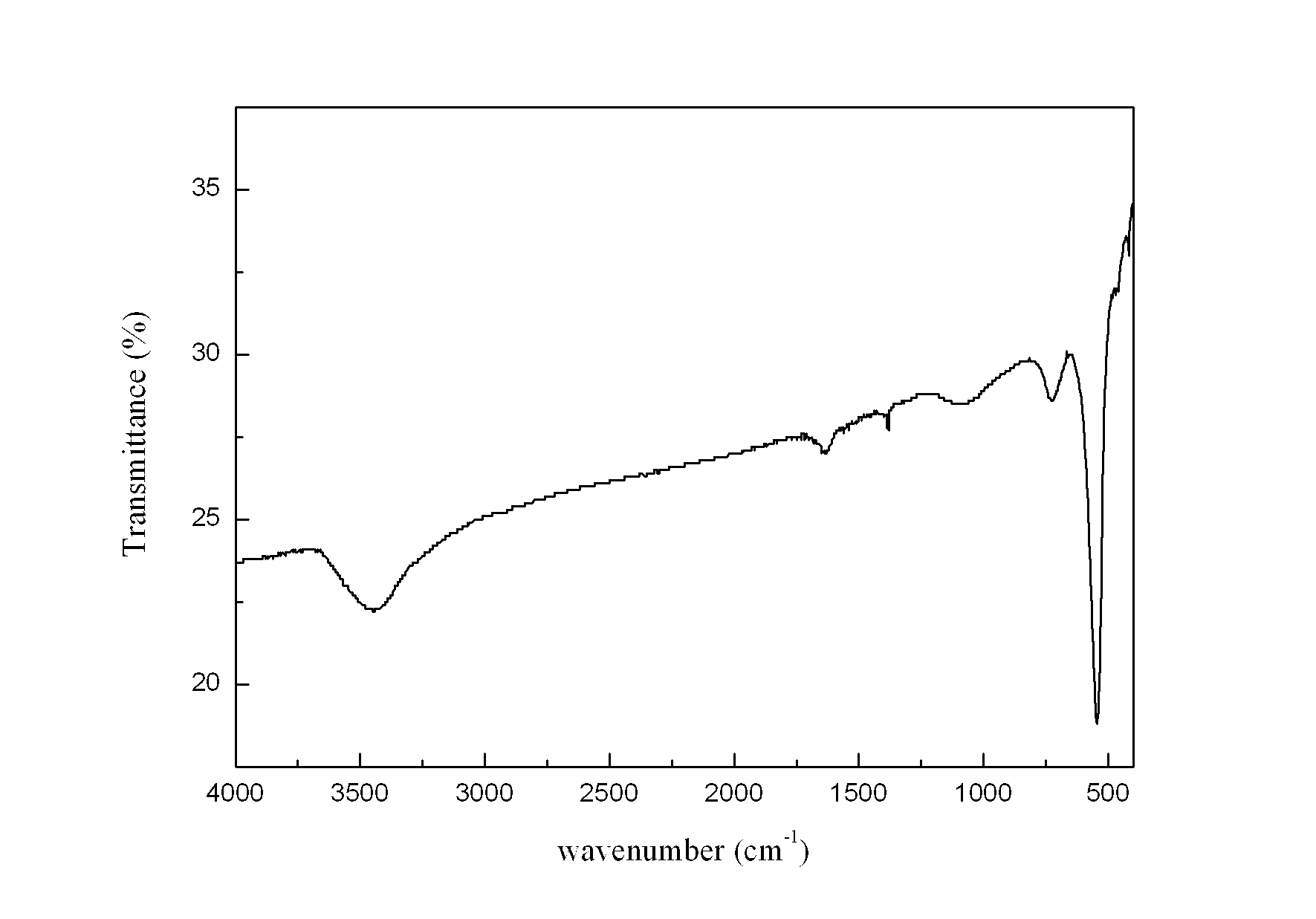
[0026] Weigh 2.8445 g KMnO 4 In a 1 L Erlenmeyer flask, add 450 mL deionized water to prepare a solution, and another 150 mL containing 0.24 mol MnSO 4 ·H 2 O and 15 mL of concentrated HNO 3 The mixed solution of KMnO was added directly 4 The solution was placed in a collector-type constant temperature heating magnetic stirrer at 100°C for reflux reaction for 36 hours, taken out and cooled naturally, then filtered, and the reaction product was washed with deionized water until the conductivity of the filtrate was less than 30 uS / cm. The product was dried in an oven at 60 °C, and its crystal structure was characterized by XRD ( figure 1 ), FTIR analysis of its main functional groups ( figure 2 ), scanning electron microscopy to characterize its morphology ( image 3 ), TEM morphology ( Figure 4 ) and the electron diffraction structure diagram ( F...
Embodiment 2
[0029] Embodiment 2: with Mn (NO 3 ) 2 Synthesis of pyrolusite (β-MnO 2 ).
[0030] Weigh 2.8445 g KMnO 4 In a 1 L Erlenmeyer flask, add 450 mL deionized water to prepare a solution, and 150 mL containing 0.24 mol Mn(NO 3 ) 2 and 15 mL concentrated HNO 3 The mixed solution of KMnO was added directly 4 Solution, placed in a collector-type constant temperature heating magnetic stirrer at 100 ° C for 36 hours of reflux reaction, taken out and filtered after natural cooling, the reaction product was washed with deionized water until the conductivity of the filtrate was less than 30 uS / cm to obtain β-MnO 2 Nano stave.
[0031]
Embodiment 3
[0032] Embodiment 3: with MnCl 2 Synthesis of pyrolusite (β-MnO 2 ).
[0033] Weigh 2.8445 g KMnO4 In a 1 L Erlenmeyer flask, add 450 mL deionized water to prepare a solution, and 150 mL containing 0.24 mol MnCl 2 and 15 mL concentrated HNO 3 The mixed solution of KMnO was added directly 4 Solution, placed in a collector-type constant temperature heating magnetic stirrer at 100 ° C for 36 hours of reflux reaction, taken out and filtered after natural cooling, the reaction product was washed with deionized water until the conductivity of the filtrate was less than 30 uS / cm to obtain β-MnO 2 Nano stave.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com