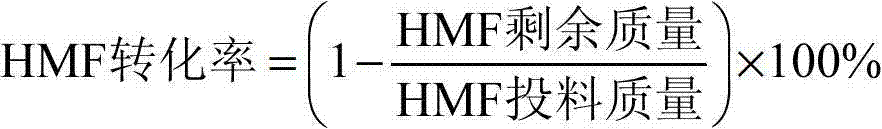Preparation method for furan-2,5-dicarbaldehyde
A technology of furandicarbaldehyde and hydroxymethylfurfural, which is applied in two fields, can solve the problems of high cost and high loading capacity, and achieve the effects of short reaction time, small investment in equipment and easy reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 0.126g of HMF, 0.05g of OMS-2 and 10mL of N,N-dimethylformamide to a 50mL reactor in sequence, seal the reactor, feed 2.0MPa oxygen, rapidly heat to 110°C, and keep for 1.5h. The reactor was cooled, the product was taken out and filtered, and the filtrate was tested to show that the HMF conversion rate was 100%, and the DFF yield was 95%; OMS-2 was washed with methanol and acetone three times, dried overnight in an oven at 120°C, and no other treatment was performed. That is to say, the conversion rate of HMF is 99% and the yield of DFF is 94% after repeated use.
[0024] The detection and calculation of the conversion rate of HMF and the yield of DFF were carried out according to the following method.
[0025] The detection of product is carried out on high-performance liquid chromatography (Shimadzu LC-20A, PDA detector; Chromatographic column is Alltech OA-1000 organic acid column; Analysis condition: 0.005mol / L H 2 SO 4 mobile phase, 0.7ml / min, 80°C). The ret...
Embodiment 2
[0033] Add 0.126g of HMF, 0.05g of OMS-2 and 2mL of dimethyl sulfoxide in sequence to a 25mL reactor, seal the reactor, inject 0.3MPa oxygen, rapidly heat to 110°C, and keep for 2.0h. The reactor was cooled, the product was taken out and filtered, and the filtrate was tested to show that the HMF conversion rate was 98%, and the DFF yield was 95%; OMS-2 was washed with methanol and acetone three times, dried overnight in an oven at 120°C, and no other treatment was performed. That is, repeated use, the HMF conversion rate is 97%, and the DFF yield is 94%.
Embodiment 3
[0035] Add 0.126g of HMF, 0.05g of OMS-2 and 5mL of N-methylpyrrolidone into a 25mL reactor in sequence, seal the reactor, feed 2.0MPa oxygen, rapidly heat to 110°C, and keep for 3.0h. The reactor was cooled, the product was taken out and filtered, and the filtrate was tested to show that the HMF conversion rate was 96%, and the DFF yield was 92%; OMS-2 was washed with methanol and acetone three times, dried overnight in an oven at 120°C, and no other treatment was performed. That is to say, the conversion rate of HMF is 94% and the yield of DFF is 90% after repeated use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com