Trithiocarbonate compound containing epoxy group, preparation method and application thereof
A technology of trithiocarbonate and epoxy group is applied in the field of epoxy group-containing trithiocarbonate compound and its preparation, which can solve the problems of inability to prepare epoxy group-containing target substances and the like, and achieves the Good application prospects, easy adjustment, good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
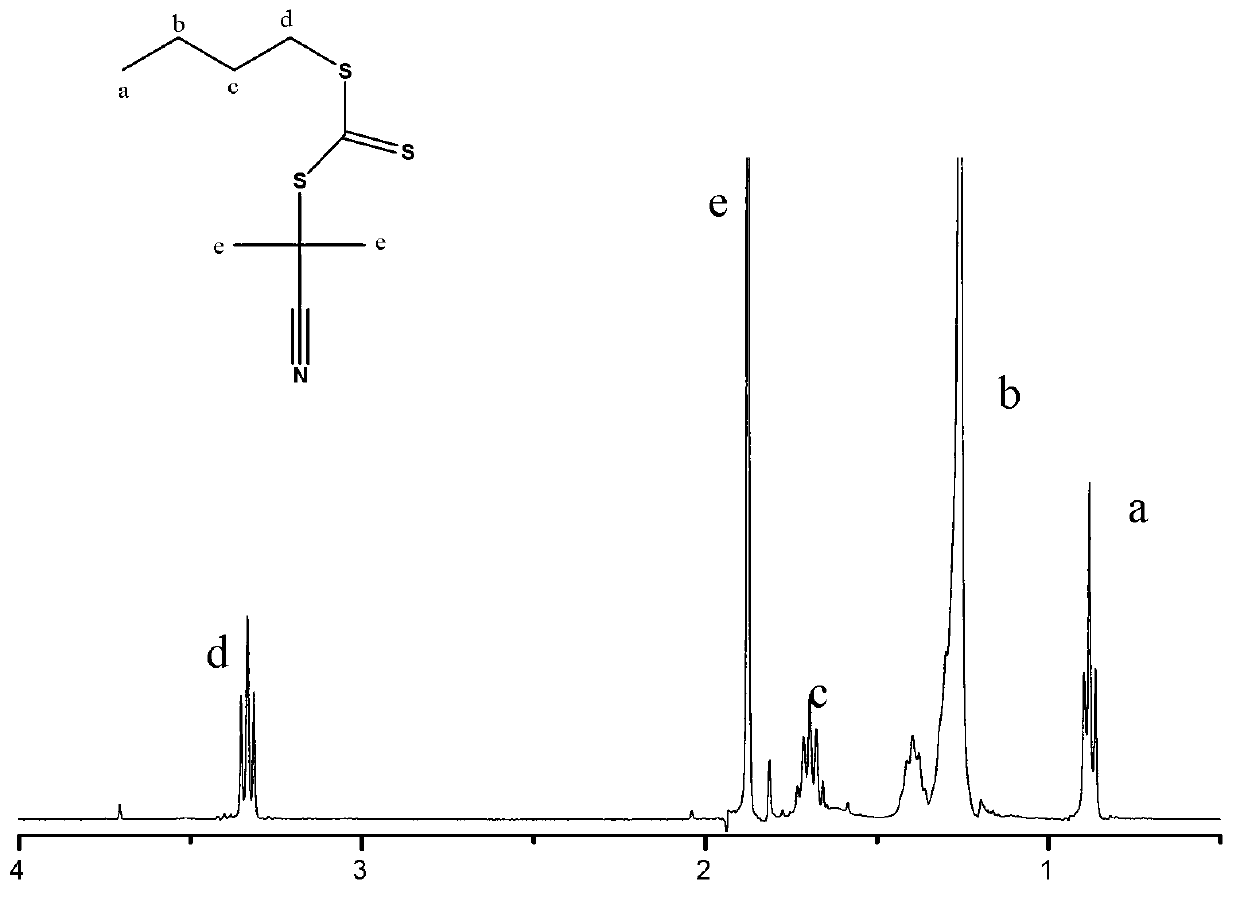
[0062] (1) Preparation of 2-(n-butyltrithiocarbonate)propanecyanide
[0063] In general, the synthesis is divided into two steps, and the reaction scheme is as follows:
[0064]
[0065] C 4 h 9 SC(S)C(S)SC 4 h 9 +AIBN→2C 4 h 9 SC(S)SC(CH 3 ) 2 CN
[0066] Dissolve 3.85g of potassium tert-butoxide (BuOK) in 19.65g of tetrahydrofuran, and pour it into a 2000ml three-necked glass reaction kettle after completely dissolving. The solvent is 1000ml of n-heptane, and slowly add 3.09g of n-butyl at 5℃~10℃. Mercaptan, stirred mechanically for 30 minutes under nitrogen protection; then slowly added 2.63g carbon disulfide with a constant pressure funnel, the process continued for 20 minutes, continued to stir for 10 minutes after the addition, and then placed at 20°C~23°C for 4h, at 16°C~18°C Add 4.34 g of solid iodine particles in batches, the process lasts about 40 min, and react at room temperature 25°C for 15 h to generate a yellow-brown reaction product.
[0067] The y...
Embodiment 2
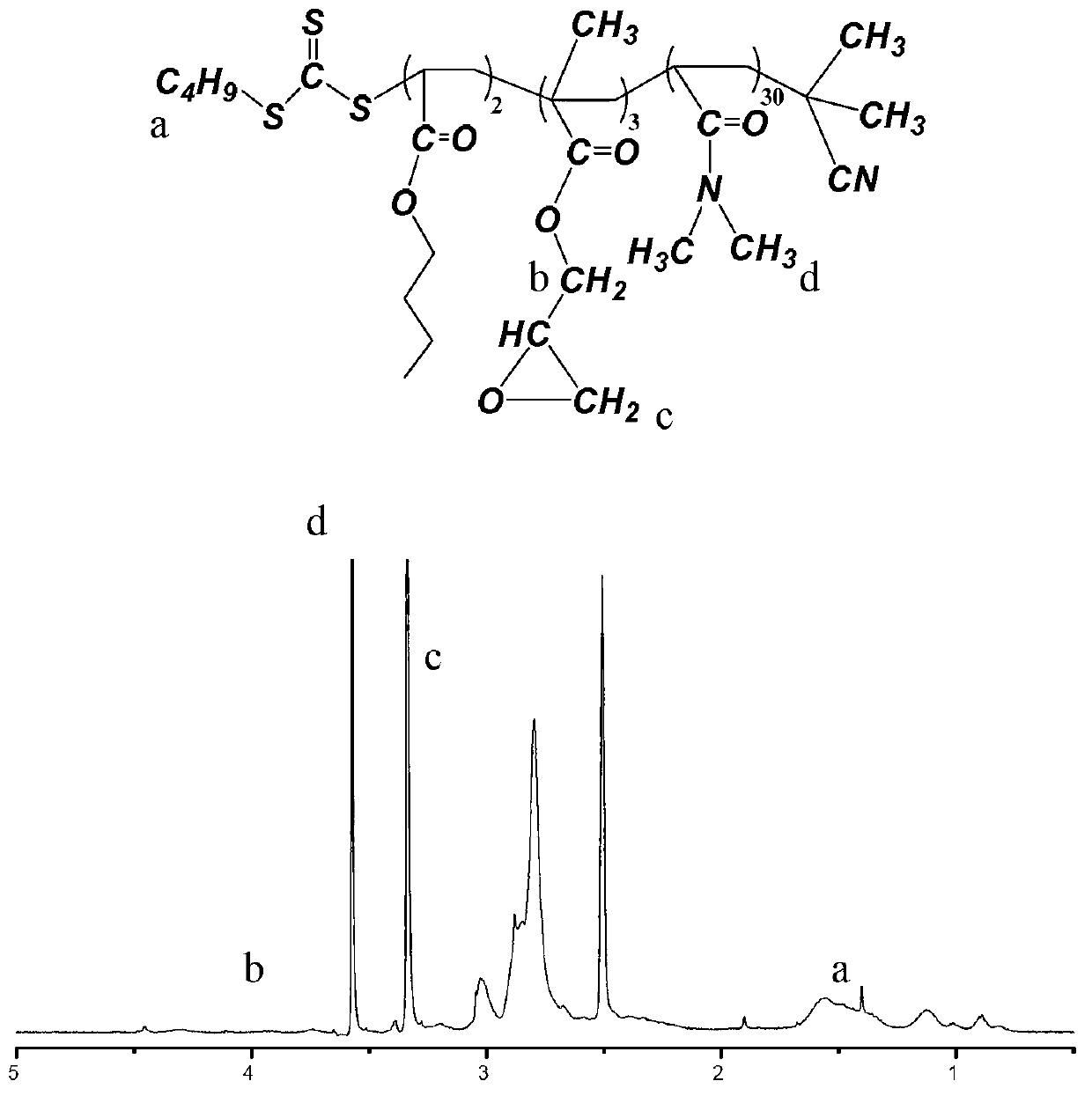
[0072] (1) Preparation of trithiocarbonate compounds containing epoxy groups.
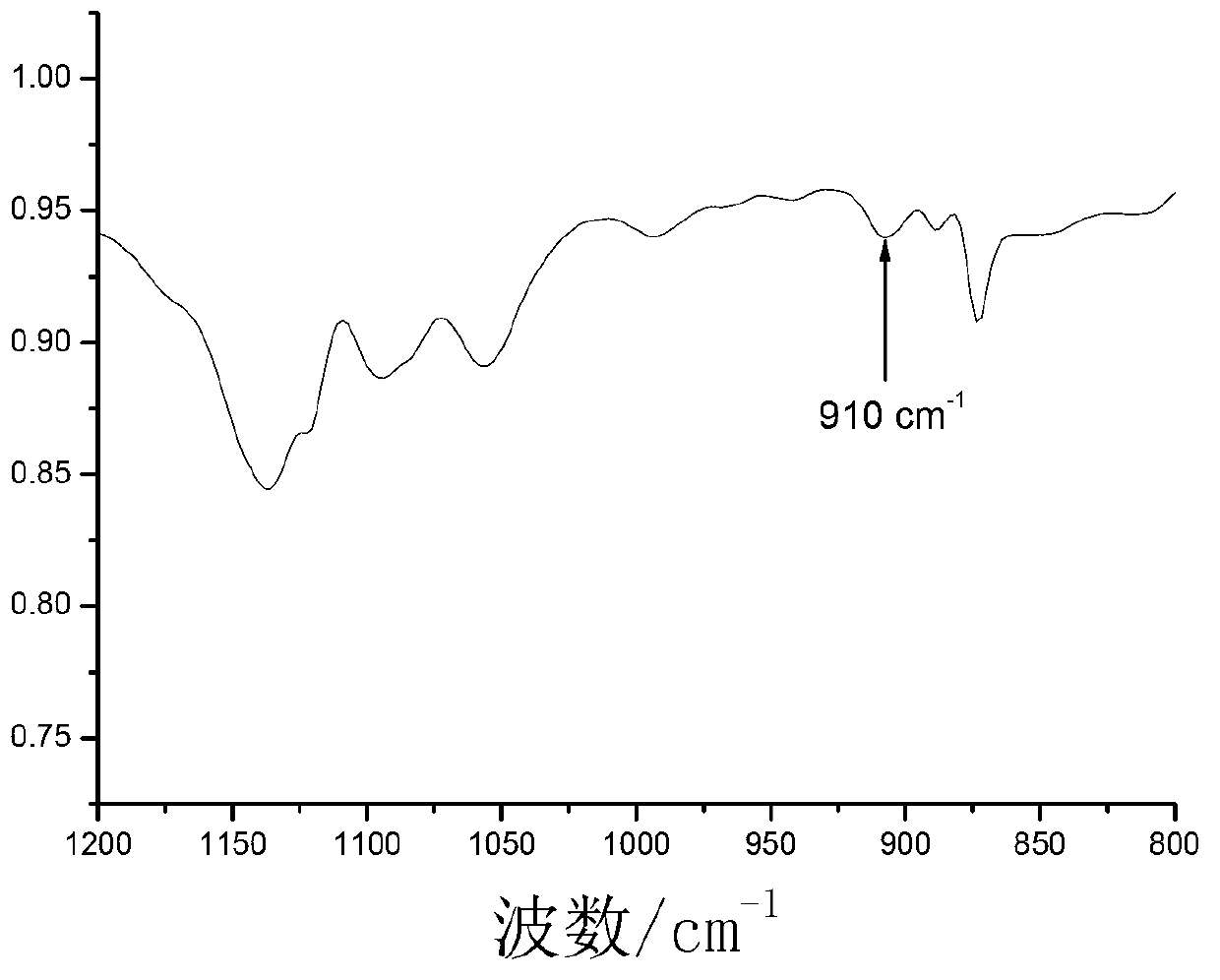
[0073] Put the following reagents into a 500mL three-necked flask equipped with a reflux condenser, a nitrogen inlet, and a feed port: 25 grams of dioxane, 1.50 grams of the product of the formula 18 structure prepared in Example 1, and 0.06 grams of azodicyanovaleric acid. g and N,N-dimethylacrylamide 13 g. After passing high-purity (99.99%) nitrogen to the above device for 1 hour, immerse in a 70°C water bath and react for 15 hours to obtain an intermediate product; continue to add 25 grams of dioxane and 0.08 grams of azobiscyanovaleric acid to the three-necked flask , 5.6 grams of monomer B containing epoxy groups with the structure of formula 13 and 3.5 grams of n-butyl acrylate with the structure of formula 15, continue to react in a 70°C water bath for 1.5 hours, then cool down, stop the reaction, and pour the product into a 200mL ring In hexane, the precipitate was collected on a Buchner f...
Embodiment 3
[0076] (1) Preparation of trithiocarbonate compounds containing epoxy groups.
[0077] In a 500mL three-necked flask equipped with a reflux condenser, a nitrogen inlet, and a feed port, the following reagents were dropped: 20 grams of dioxane, 0.54 grams of the product of the formula 18 structure prepared in Example 1, and 0.03 grams of azodicyanovaleric acid. g and N,N-dimethylacrylamide 7.0 g. After passing high-purity (99.99%) nitrogen to the above device for 1 hour, immerse in a 70°C water bath and react for 15 hours to obtain an intermediate product; continue to add 10 grams of dioxane and 0.08 grams of azodicyanovaleric acid to the three-necked flask 1.026 grams of epoxy-group-containing monomers of formula 12 and 0.576 grams of n-butyl acrylate of formula 15 were reacted in a water bath at 70°C for 2 hours, then the temperature was lowered to stop the reaction, and the product was poured into 200 mL of cyclohexane The precipitate was collected with a Buchner funnel. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com