Polyamino acid segmented copolymer serving as siRAN carrier and preparation method as well as composite particle
A technology of block copolymer and polyamino acid, applied in the field of polymer, can solve the problems of insufficient concentration of charge density and weak ability to compound genetic material, and achieve the effects of reducing toxicity, good biocompatibility, and strong compounding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
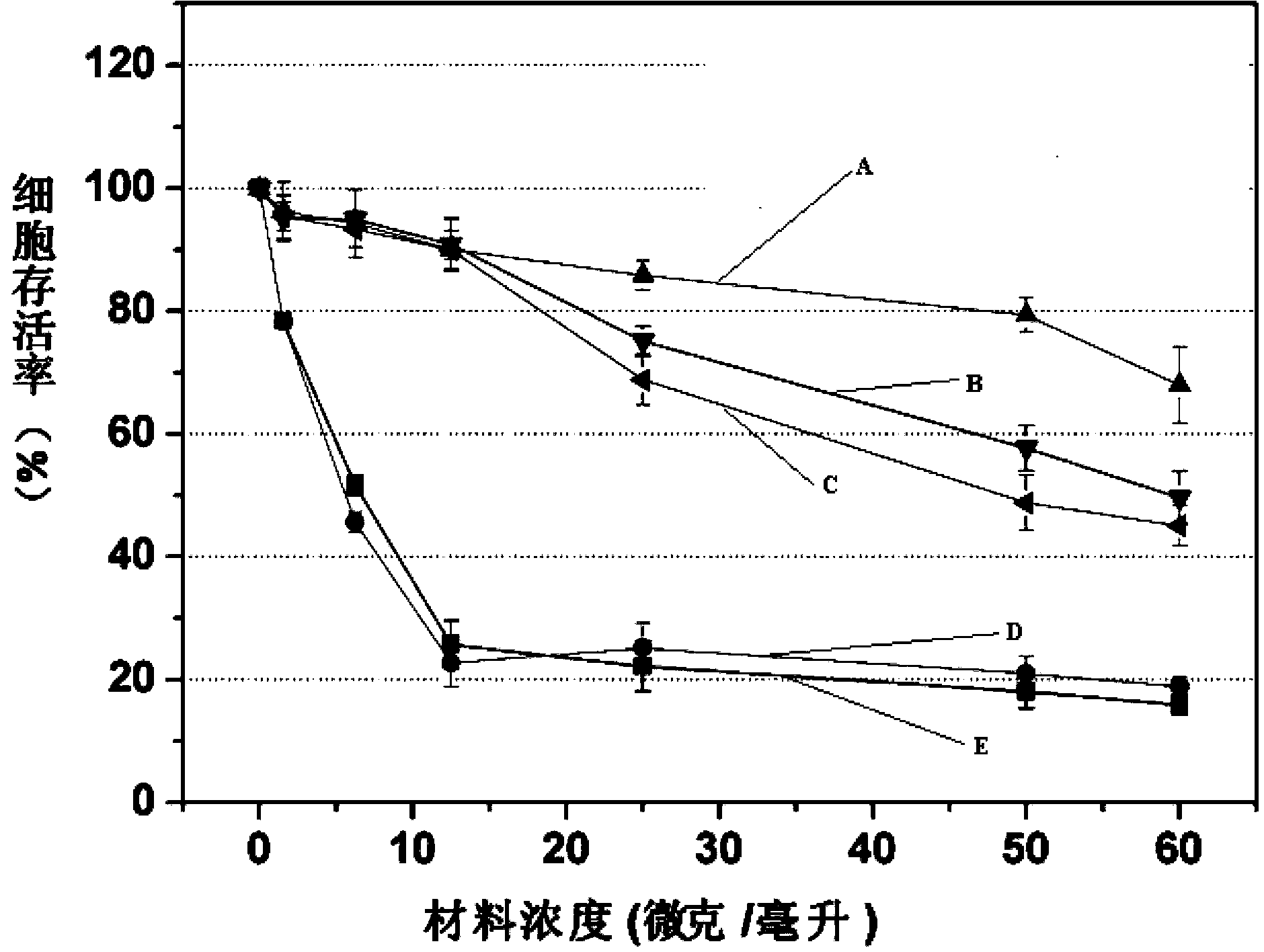
Examples
preparation example Construction
[0048] The invention also discloses a preparation method of a polyamino acid block copolymer used as an siRNA carrier, comprising the following steps:
[0049] (A) Under the protection of inert gas, β-benzyl-L-aspartic acid-N-carboxylic acid anhydride reacts with branched polyethyleneimine in an organic solvent to obtain an intermediate product;
[0050] (B) reacting the intermediate product obtained in step (A) with polyethyleneimine under the action of a catalyst to obtain a polyamino acid block copolymer used as an siRNA carrier;
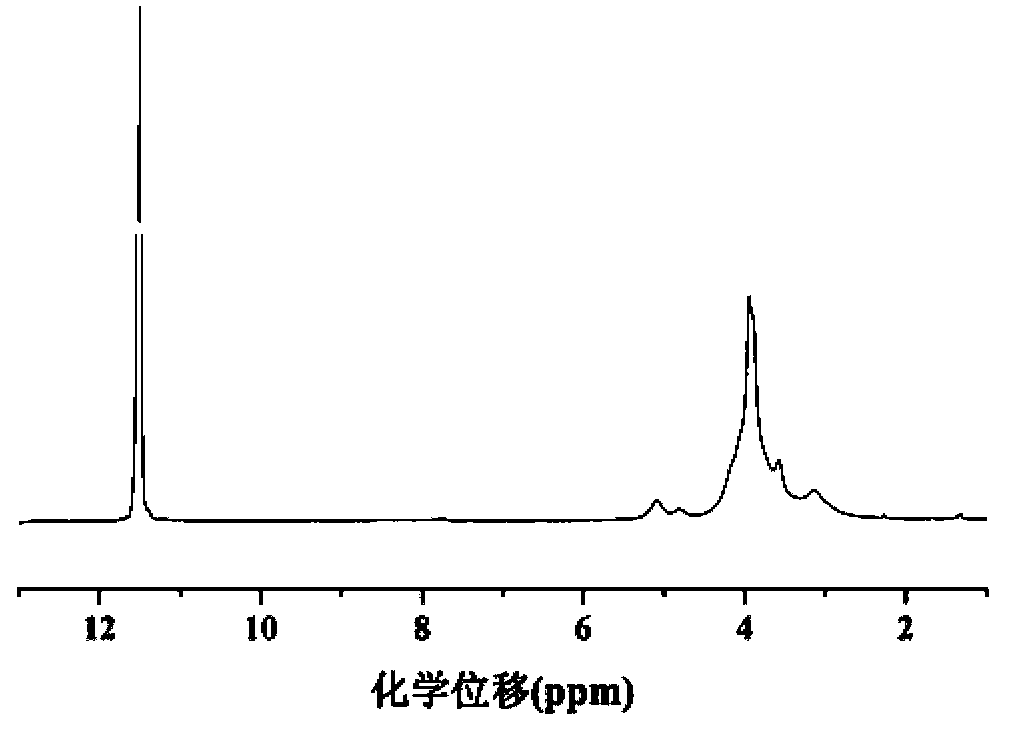
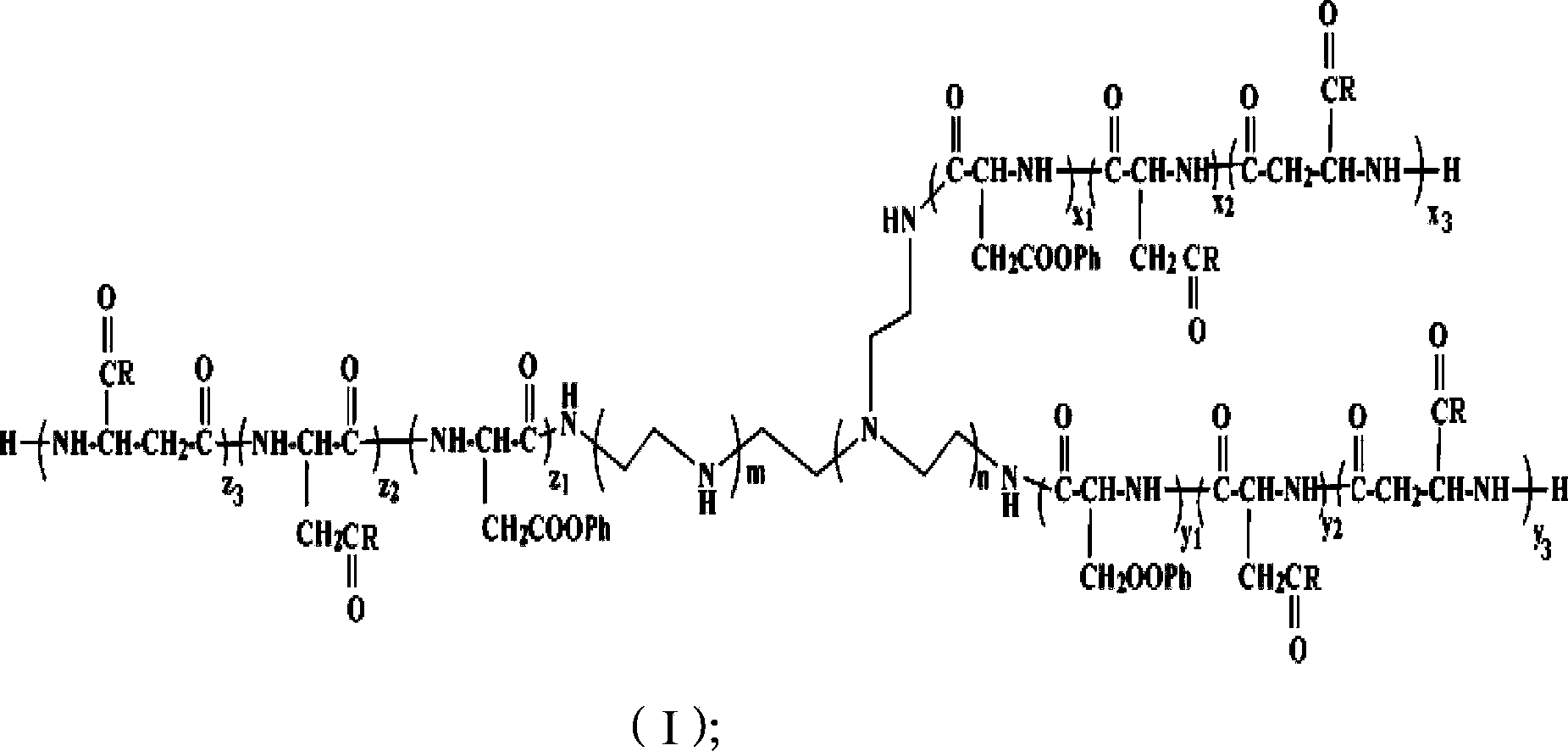
[0051] The polyamino acid block copolymer used as siRNA carrier is shown in formula (I):
[0052]
[0053] R is selected from one of the structures shown in formula (11) ~ formula (14):
[0054]
[0055]
[0056] The NH- of the R is connected to the C atom in the formula (I);
[0057] Ph is
[0058] where x 1 ,y 1 ,z 1 ,x 2 ,y 2 ,z 2 ,x 3 ,y 3 ,z 3 , m, n, a, b and c are degrees of aggregation, x 1 ,y 1 ,x 3 ,y 3 ,z 3 ...
Embodiment 1
[0070] Under anhydrous and oxygen-free conditions, add 4.7 g of β-benzyl-L-aspartic acid-N-carboxylic acid anhydride to a dry reaction ampoule, fill it with nitrogen protection, and add 300 mL of anhydrous N',N' - Dimethylformamide, stirred to dissolve.
[0071] 19 mL of branched polyethyleneimine with a weight average molecular weight of 600 was dissolved in anhydrous chloroform to obtain a polyethyleneimine / chloroform solution with a concentration of 0.01 mmol / mL. Take 19 mL of polyethyleneimine / chloroform solution and add it into the reaction ampoule, and stir the reaction at 30° C. for 72 hours under temperature control. After the reaction is completed, settle in 3000 mL of ether, filter and dry the filter cake to obtain poly-L-aspartic acid-β-benzyl ester with a molecular weight of 21000.
Embodiment 2
[0073] Under anhydrous and oxygen-free conditions, add 4.7 g of β-benzyl-L-aspartic acid-N-carboxylic acid anhydride to a dry reaction ampoule, fill it with nitrogen protection, and add 300 mL of anhydrous N',N' - Dimethylformamide, stirred to dissolve.
[0074]3.8 mL of branched polyethyleneimine with a weight average molecular weight of 600 was dissolved in anhydrous chloroform to obtain a polyethyleneimine / chloroform solution with a concentration of 0.01 mmol / mL. Take 19 mL of polyethyleneimine / chloroform solution and add it into the reaction ampoule, and stir the reaction at 30° C. for 72 hours under temperature control. After completion of the reaction, settle in 3000 mL of ether, filter and dry the filter cake to obtain poly-L-aspartic acid-β-benzyl ester with a molecular weight of 100800.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com