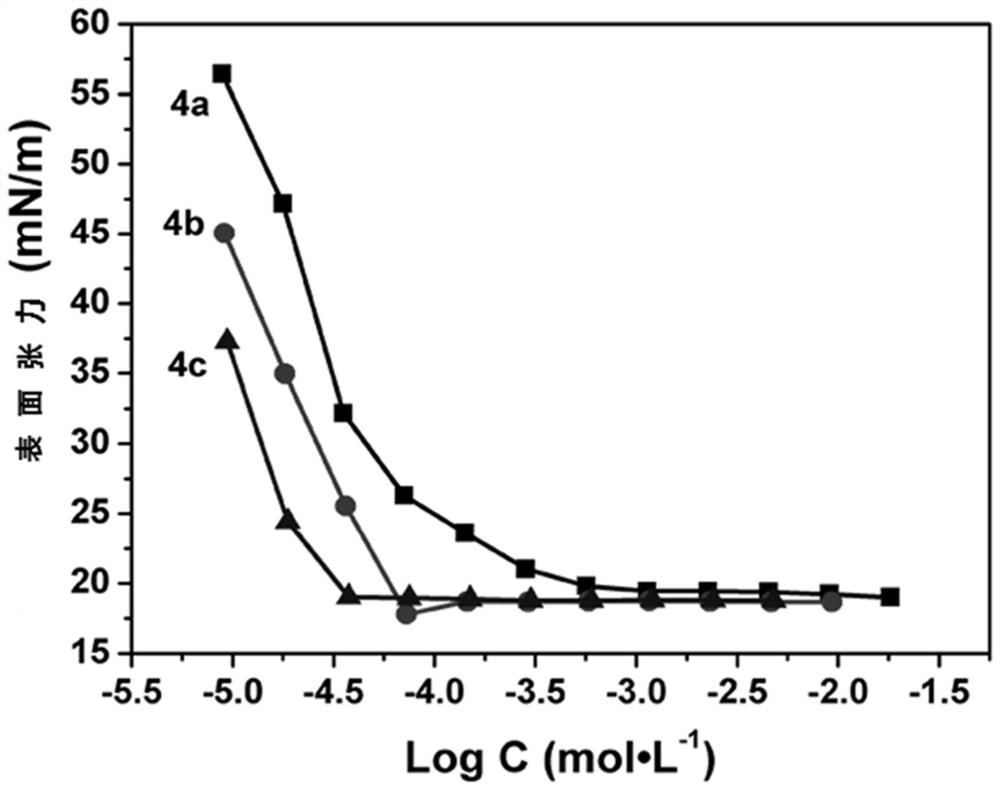
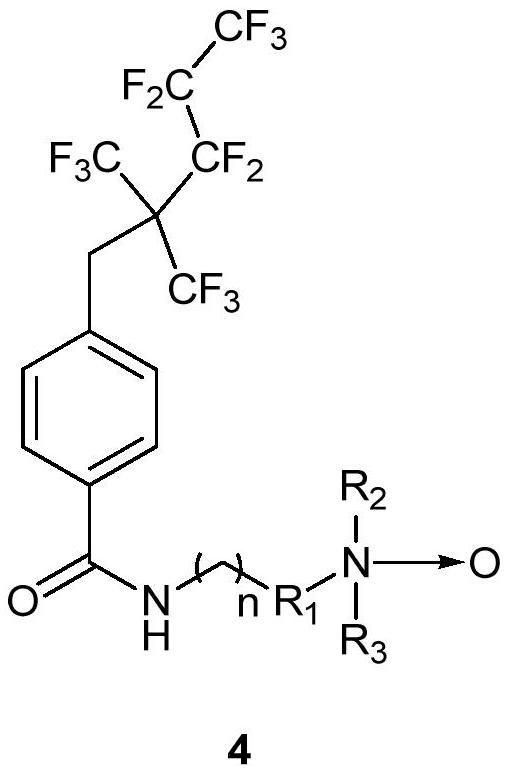
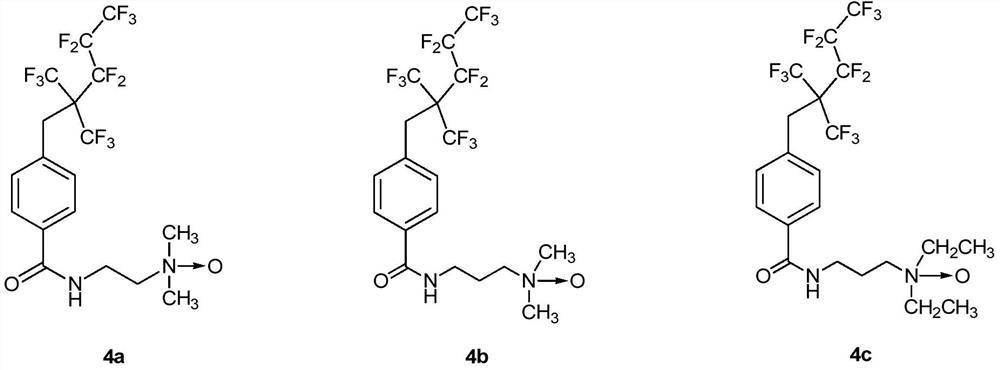
A kind of amphoteric fluorine-containing surfactant and its preparation method and application
A solvent and oxidant technology, applied in chemical instruments and methods, fire protection equipment, transportation and packaging, etc., can solve the problems of difficult degradation of fluorocarbon surfactants, difficult synthesis of fluorocarbon surfactants, high price, etc., and achieve production costs Low, strong recombination, and the effect of reducing surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The synthesis of embodiment 1 fluorine-containing intermediate compound 1
[0094] 6.00g (39.5mmol, 1.5equiv) cesium fluoride, 6.00g (26.3mmol, 1.0equiv) methyl p-bromomethylbenzoate, 15.78g (52.6mmol, 2.0equiv) perfluoro-2-methyl-2 -Pentene, 0.08g (0.26mmol, 0.01equiv) tetrabutylammonium bromide and 40.0mL of anhydrous acetonitrile were sequentially added to a 100mL sealed tube, sealed and stirred at 50°C for 2 days. The mixed system was poured into ether, and the organic phase was first washed twice with deionized water, then washed with saturated brine, and finally dried with anhydrous sodium sulfate, filtered, and the solvent removed. The mixture was separated by EA:PE=1:48 eluent column chromatography to obtain 11.69 g of fluorine-containing intermediate compound 1 with a yield of 95%.
[0095] 1 H NMR (CDCl 3 ,300MHz)δ:3.58(s,2H),3.91(s,3H),7.37(d,J=8.4Hz,2H),7.98(d,J=8.4Hz,2H). 19 F NMR (CDCl 3 ,282MHz)δ:-62.65~-62.85(m,6F),-80.57(t,J=13.7Hz,3F),-106.20~-106...
Embodiment 2
[0096] Synthesis of embodiment 2 fluorine-containing intermediate compound 2
[0097] 2.34g (5.0mmol, 1.0equiv) of fluorine-containing intermediate compound 1 was dissolved in 50.0mL of tetrahydrofuran, and 12.0mL (6.0mmol, 1.2equiv) of 0.5mol / L aqueous sodium hydroxide solution was added dropwise in an ice-water bath. After dropping, the mixed system was moved to room temperature for 12 h. Add deionized water to the system, wash with ether, and adjust the water phase to pH=3 with 1 mol / L HCl solution in an ice-water bath. After the aqueous solution was extracted three times with ether, the ether phases were combined, dried with anhydrous sodium sulfate, filtered, and the solvent was removed to obtain 2.25 g of fluorine-containing intermediate compound 2 with a yield of 99%.
[0098] 1 H NMR (DMSO-d6, 300MHz) δ: 3.82(s, 2H), 7.45(d, J=7.8Hz, 2H), 7.92(d, J=7.8Hz, 2H). 19 F NMR (DMSO-d6, 282MHz) δ: -61.95~-62.20(m, 6F), -80.04(t, J=13.1Hz, 3F), -106.00~-106.30(m, 2F), -122.9...
Embodiment 3
[0099] Embodiment 3: the synthesis of fluorine-containing intermediate compound 3a
[0100] Add 5.00g (11.0mmol, 1.0equiv) compound 2, 0.14g (1.1mmol, 0.1equiv) DMAP and 3.15g (16.5mmol, 1.5equiv) 1-ethyl-(3-di Methylaminopropyl) carbodiimide hydrochloride (EDCI), after pumping and exchanging gas three times, add 50.0 mL of anhydrous dichloromethane under ice-water bath, and stir for 10 min. Add 1.8mL (16.5mmol, 1.5equiv) N,N-dimethylethylenediamine dropwise to the system, and after the drop is complete, the system is moved to room temperature for reaction. The reaction process was monitored by TLC, and the reaction was completed after 5 h. The system was washed successively with deionized water and saturated brine. The organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent was removed. Finally, the mixture was separated by column chromatography to obtain 5.13 g of fluorine-containing intermediate compound 3a, with a yield of 89%.
[0101] 1 H NM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com