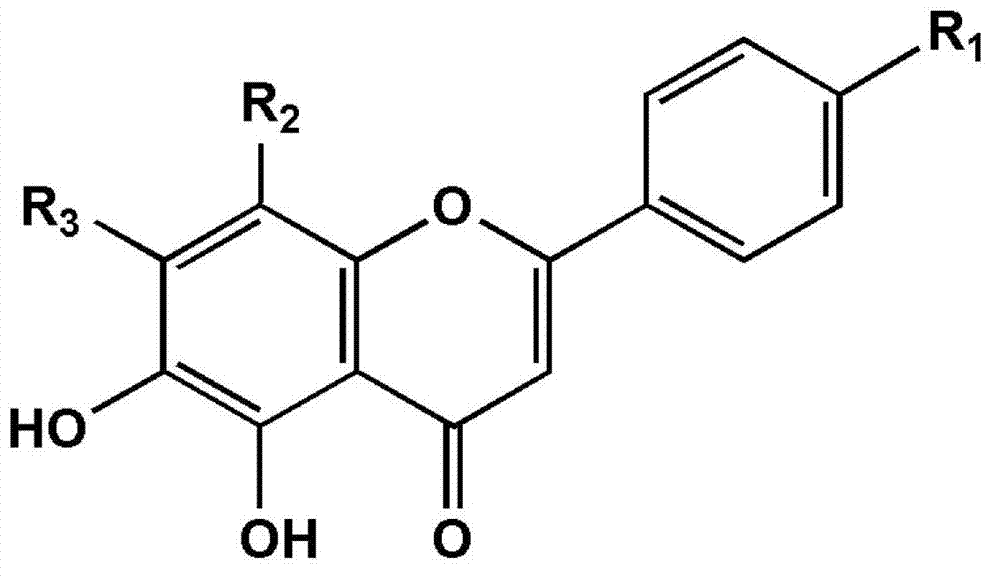
Application of compound with flavone skeleton structure as Parkinsonism treating medicine
A Parkinson's disease and skeleton structure technology, applied in the field of medicine, can solve problems such as differences in substrate affinity, and achieve high inhibitory activity and high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
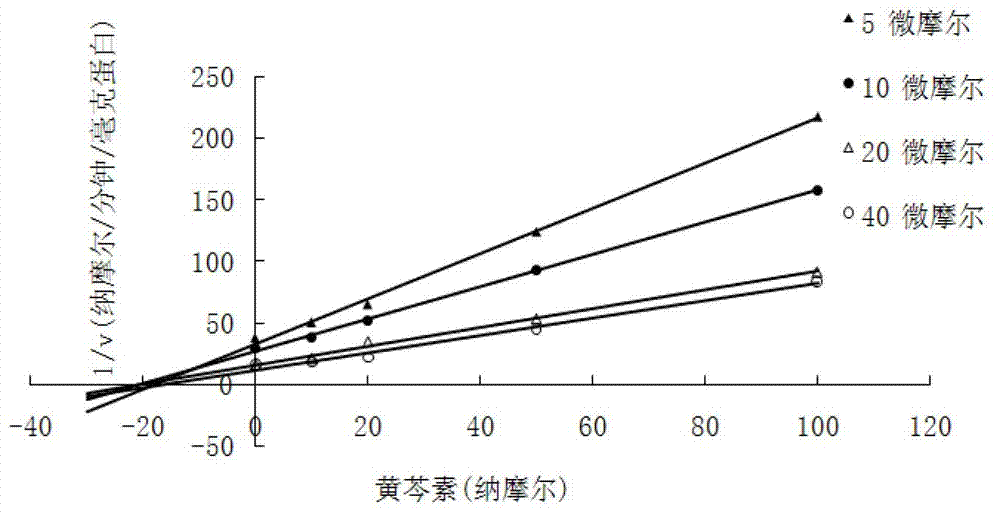
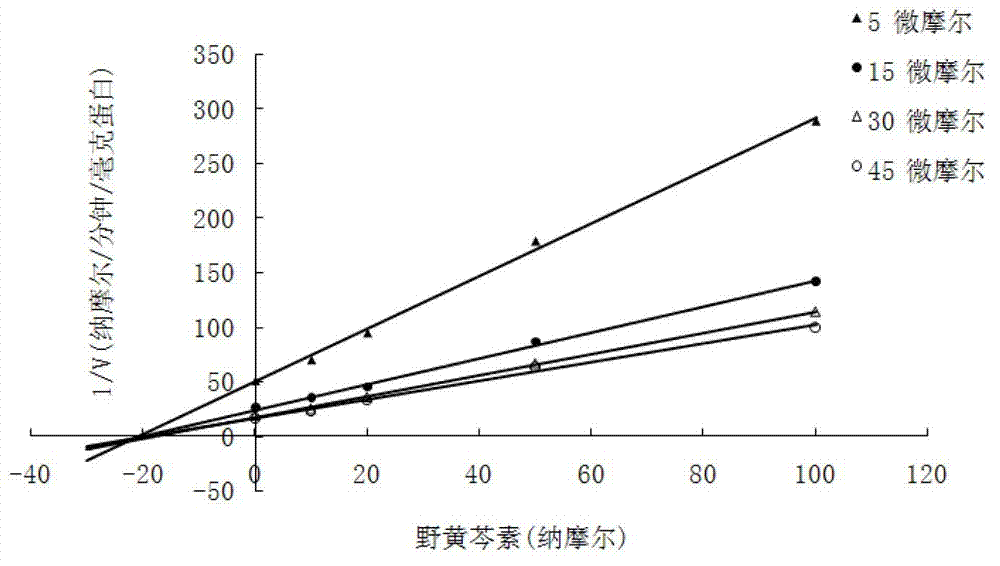
[0019] Embodiment 1. Flavonoid catechol series compound is to the inhibitory activity determination of COMT enzyme
[0020] Using the methylation reaction of protocatechuic acid as a probe reaction, the IC of COMT enzyme inhibition by catechol series compounds was determined by means of human liver cell plasma in vitro incubation system 50 , the specific experimental procedure is as follows:
[0021] (1) Add 5mM MgCl to 200 microliters of in vitro metabolic reaction system 2 , 2mM dithiothreitol, 0.2mM S-adenosylmethionine, human liver cell plasma protein concentration is 1mg / ml, inhibitor final concentration range is 0.01μM-1μM, pre-incubated at 37°C for 3 minutes;
[0022] (2) Add the substrate (final concentration 15uM) to the reaction system to initiate the reaction; after reacting at 37°C for 30 minutes, add 200 μl of acetonitrile and shake vigorously to terminate the reaction;
[0023] (3) Using a high-speed refrigerated centrifuge, under the condition of 20,000×g, cen...
Embodiment 2
[0027] Example 2. Evaluation of metabolic activation of flavonoid catechol series compounds
[0028] In the human liver microsome incubation system with cofactor NADPH or its production system, add each flavonoid catechol series compound (final concentration: 100μM) and reduced glutathione (final concentration: 1000μM), 37℃ After incubation for 120 minutes, the reaction was terminated with an equal volume of acetonitrile, the protein was removed by centrifugation, and the adduct was detected by AB Qtrap5500 to detect whether an active adduct was captured. The results are shown in Table 2.
[0029] Table 2
[0030]
[0031] It can be seen that only the marketed drug tolcapone can produce active adducts, while no active adducts have been detected in flavonoid catechol series compounds. The results suggest that this type of compound will not produce active intermediates through metabolic activation, and will not form toxic adducts with biological macromolecules, so it will n...
Embodiment 3
[0032] Example 3. The overall pharmacokinetic study of scutellarein combined with L-dopa
[0033] Select 18 Wistar rats, half male and half male, and randomly divide them into 3 groups according to body weight (180-220g), 6 rats / group carry out the overall pharmacokinetic study of oral administration of L-dopa alone and simultaneous oral administration of scutellarein and L-dopa , and compared with the effect of the positive control (tolcapone) on the pharmacokinetics of L-dopa. About 0.5ml of rat plasma samples were collected before administration and at 5, 10, 15, 30, 60, 120, 180, and 240 minutes after administration, and placed in pre-heparinized 1.5ml pointed-bottomed brown centrifuge tubes , after centrifugation at 4000×g for 10 minutes, separate the plasma, add an equal volume of methanol to precipitate protein and centrifuge at high speed (20,000×g), remove the supernatant and store it in a -80°C refrigerator for testing. UFLC-ESI-MS was used to measure the blood conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com