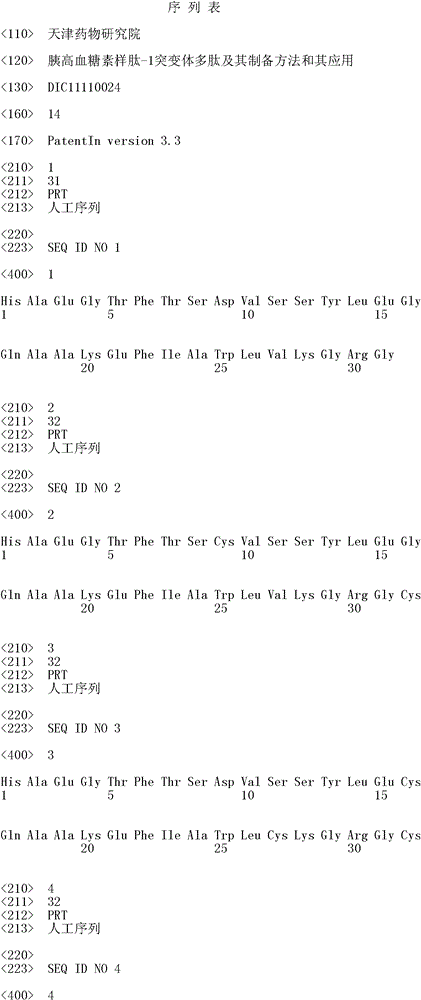Glucagon-like peptide-1 mutant polypeptide, preparation method and application thereof
A technique for glucagon and mutants, applied in the field of novel glucagon-like peptide-1 (GLP-1) mutant polypeptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Synthesis of embodiment 1 GLP-1 mutant tandem polypeptide
[0099] 1.1: Determination, preparation and sequencing verification of the GLP-1 polypeptide sequence. Using the amino acid protected by Fmoc at the amino terminal as the raw material, it was synthesized by the solid-phase synthesis method on the Liberty1 single-channel fully automatic microwave peptide synthesis system (purchased from the Beijing office of Paian Technology Co., Ltd., USA). The synthesis method refers to the manufacturer's instrument manual. conduct. After deprotection, coupling and final cleavage of Fmoc amino acids (purchased from Shanghai Jier Biochemical), the target polypeptide is finally formed. The obtained polypeptide was confirmed by sequencing by Shanghai Sangon Biotechnology Company.
[0100] 1.2: Exendin-4 31-39 identification, preparation and sequencing verification. Using the amino acid protected by Fmoc at the amino terminal as the raw material, it was synthesized by the sol...
Embodiment 2
[0104] Example 2 GLP-1 mutant tandem polypeptide GLP-1 receptor binding experiment in vitro
[0105] The receptor binding experiments were performed using the GLP-1 receptor high-expressing cell line INS-1 (rat β-insulinoma cells, ATCC, Manassas, VA). First, INS-1 cells were seeded on a 12-well plate, and the cells were washed with PBS buffer 2 hours before the experiment, and then the cells were fixed with 4% paraformaldehyde for 10 minutes at room temperature. After cell counting, 10 5 Each cell / well was co-incubated with GLP-1 mutant tandem polypeptide (SEQ ID NO6-14) and natural GLP-1 for 2 hours respectively. After washing the unbound polypeptides with PBS, the GLP-1 ELISA kit was used for detection. It can be seen from Table 1 that the GLP-1 mutant tandem polypeptide greatly improves its binding ability to the GLP-1 receptor, compared with GLP-1.
[0106] Table 1. Binding constants of GLP-1 mutant tandem polypeptides and GLP-1 polypeptides binding to GLP-1 receptors...
Embodiment 3
[0108] Example 3 Experiment of hypoglycemic function of GLP-1 mutant tandem polypeptide in mice
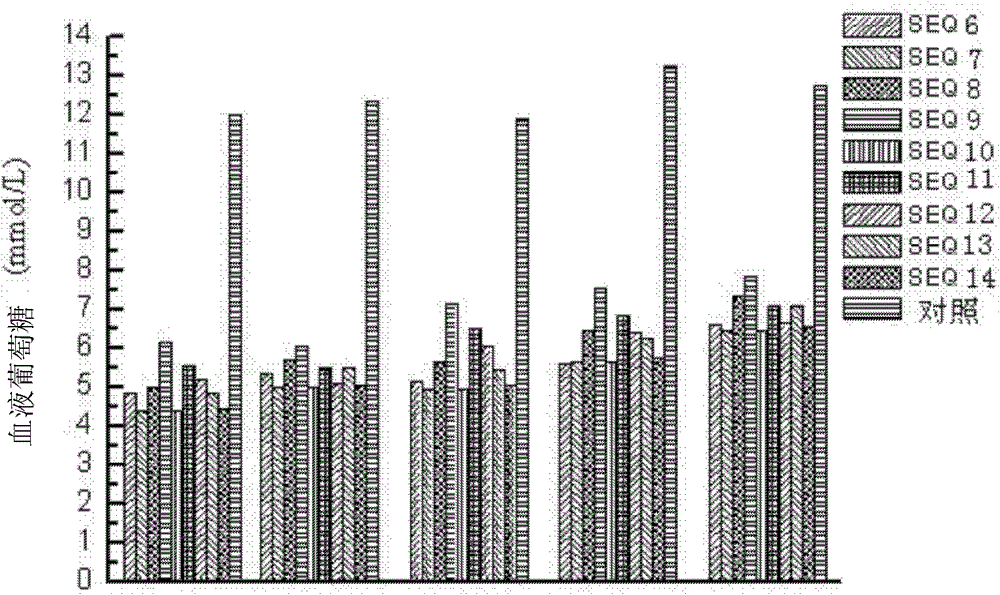
[0109] Intravenously inject mice with 9 different GLP-1 mutant tandem polypeptides described in the present invention (injection dose is 100 μg / kg), with Exendin-4 as a positive control, at 0.5, 4, 6, and 8 hours after administration, respectively, , 12, 24 and 48 hours inject glucose (2g / kg), measure the blood glucose level half an hour after the injection, from figure 1 It can be seen that Exendin-4 completely loses its hypoglycemic function after 4 hours, but the nine GLP-1 mutant tandem peptides still have the hypoglycemic function after 48 hours of administration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com