Inorganic phosphate compositions and methods
A phosphate and composition technology, applied in the field of multi-component inorganic phosphate products, can solve the problems of low viscosity, difficulty in maintaining large aggregates, and taking too long to cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
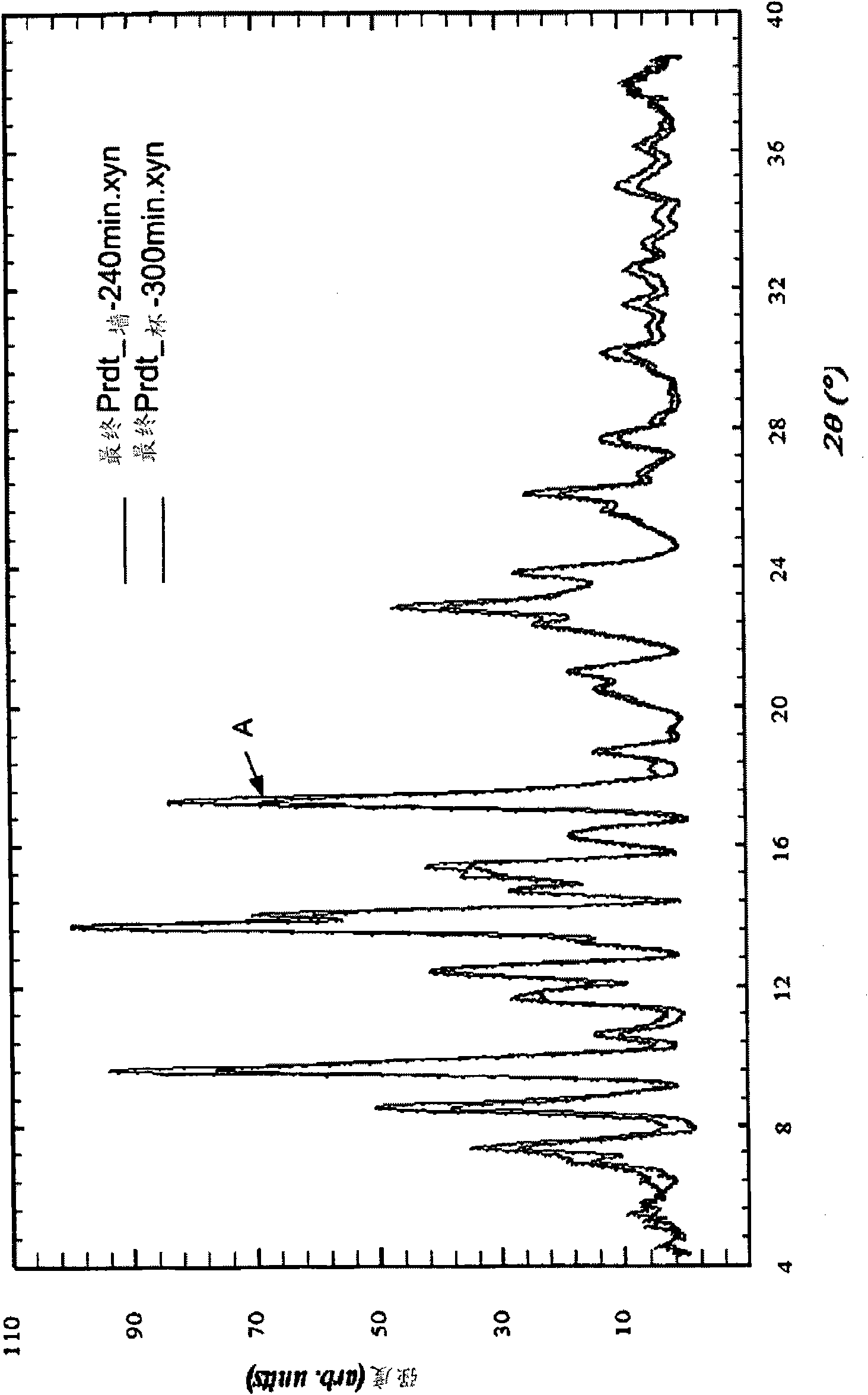
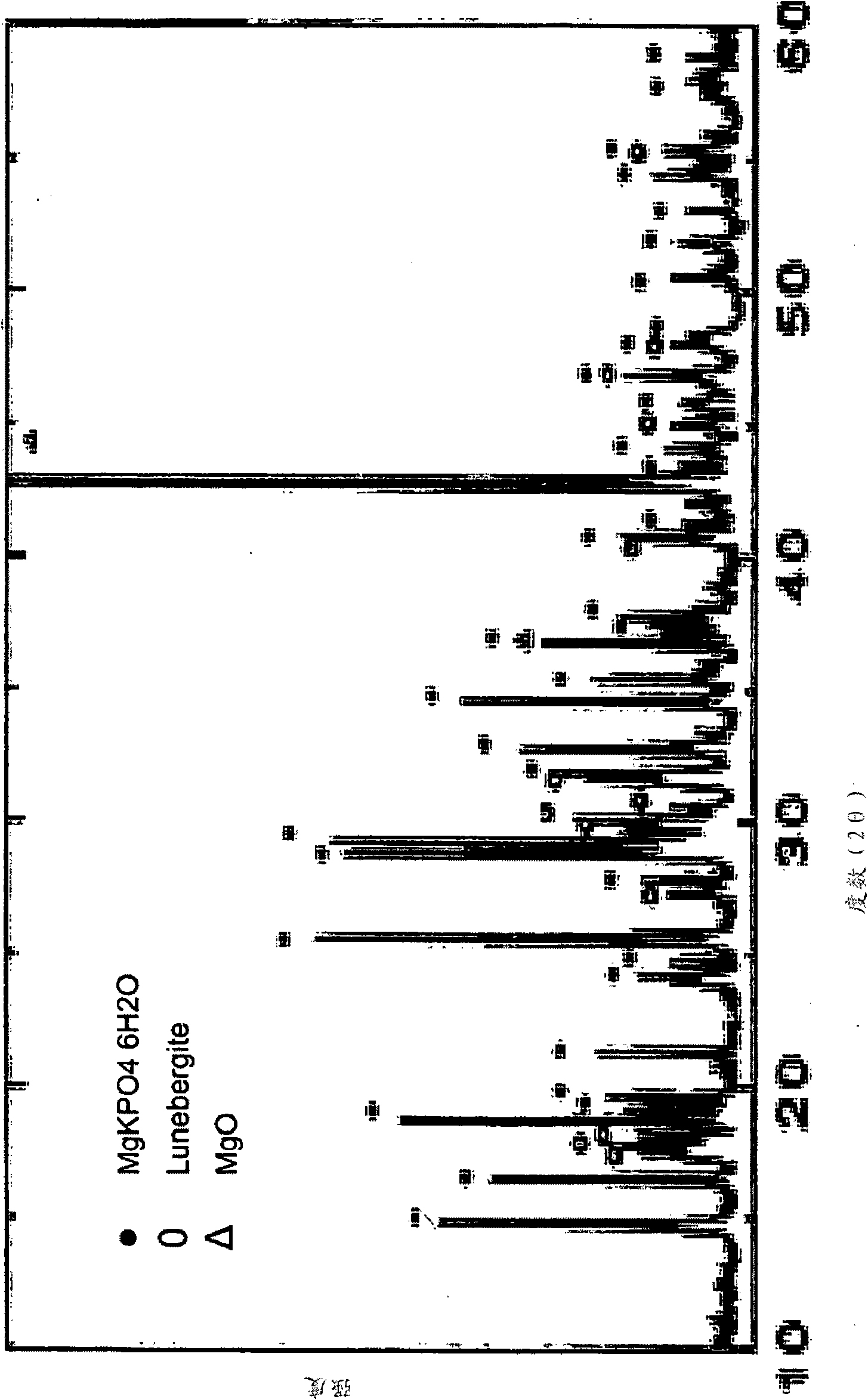
[0125] Example 1. Magnesium Potassium Phosphate Composition
[0126] In this experiment, the first (acidic) component consisted of monopotassium phosphate (MKP) having a pH value of about 4.2 when measured in aqueous solution. The MKP was milled for one hour to about 74 micron size powder (US 200 sieve) before use. The MPK was then further milled for one hour and its pH was adjusted between 3.2 and 3.5 by adding 2.5% by weight phosphoric acid (added as a 50% dilute solution). 0.5% by weight copper oxide was added to prevent algae growth. The solution was adjusted with water to produce a viscosity of approximately 10,000 centipoise. The component has a density of 1.9 g / cm 3 .
[0127] The second component (basic) was prepared as follows: The required amount of magnesium brine solution (Martin Marietta) as a source of magnesium hydroxide was weighed into a mixer. Zr(OH) 4 The powder was added to the brine and stirred for 5-10 minutes until homogeneous. Wollastonite passin...
Embodiment 2
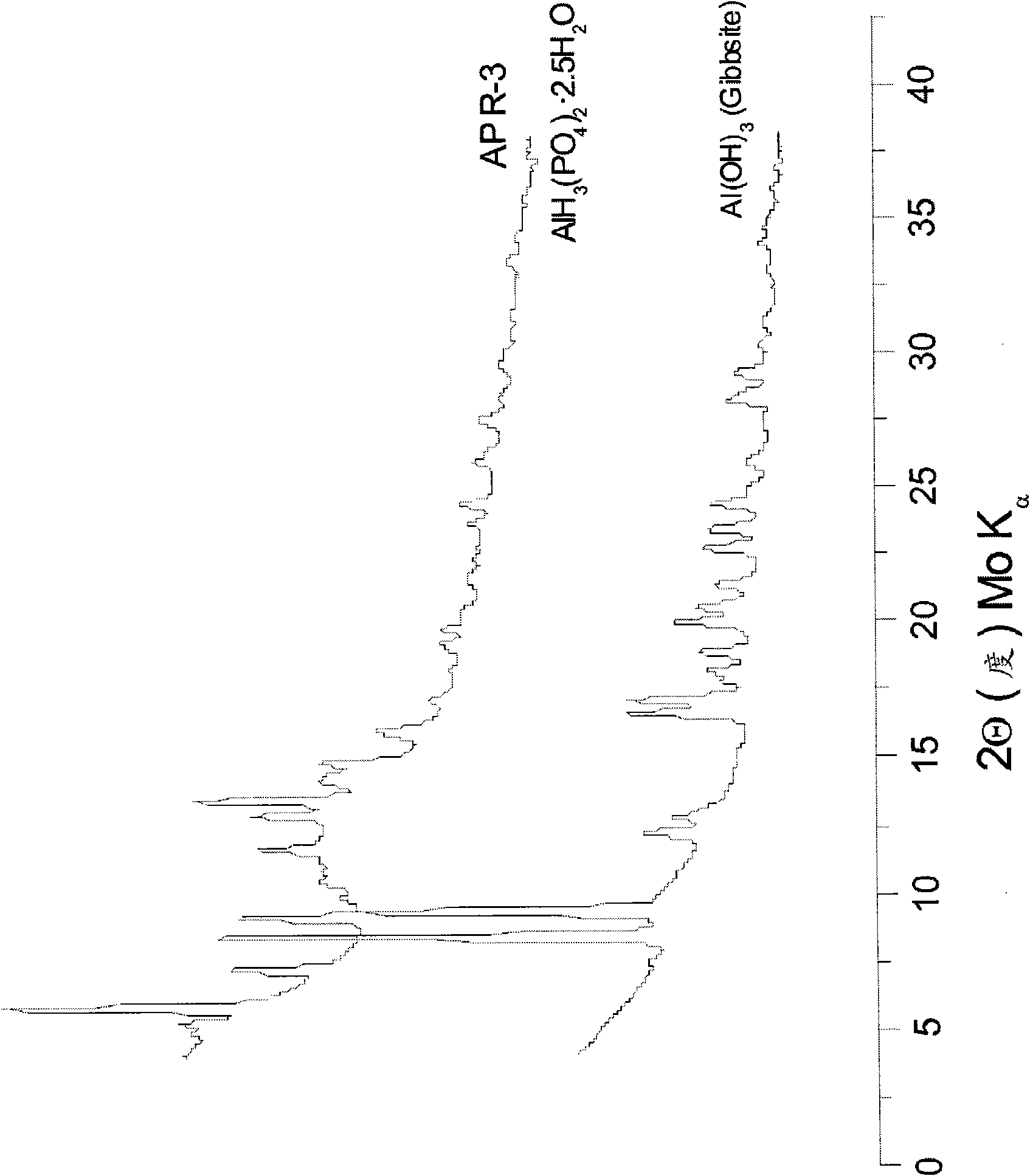
[0137] Example 2. Aluminum Phosphate Composition
[0138] 30g aluminum hydroxide (gibbsite, Al(OH) 3 ) was added to 173.4g phosphoric acid solution (H 3 PO 4 , 50% by weight, molar ratio Al(OH) 3 :H 3 PO 4 =1:2.3). 1.5 g (5% by weight) of potassium fluoride and 1.5 g of potassium permanganate were added to the Al(OH) 3 in the acid together. The mixture is stirred at 100-110°C for 60 minutes to about 3 hours. The product obtained after cooling to room temperature was a viscous slurry. This is labeled Component A.
[0139] Potassium permanganate as an exemplary oxidizing agent was added as an optional reagent to reduce hydrogen gas formation in Example 2. The use of an oxidizing agent provides improved adhesion to surfaces such as steel / iron surfaces. The results of the coating of the composition of Example 2 on the steel / substrate provided improved adhesion between the inorganic phosphate composition and the metal compared to its adhesion in the absence of an oxidizi...
Embodiment 3
[0146] Example 3: Method of Forming a Phosphorite Coating
[0147] Theoretical analysis based on thermodynamic principles shows that aluminum trihydrogen phosphate, if combined with alumina (corundum, Al 2 o 3 ) reaction, aluminum phosphate (AlPO 4 ) (block phosphate aluminite). The azophosphate mineral phase, which is stable up to 1500°C, will provide high temperature coatings. Therefore, 100 grams of aluminum trihydrogen phosphate (AlH 3 (PO 4 ) 2 -5H 2 O) Viscous slurry was mixed with 50 grams of alumina fine powder and mixed well to form a viscous slurry. Brush it on a mild steel substrate preheated at 175°C. First, some of the water of the slurry partially evaporates, but then the coating bonds well to the steel. The entire assembly was maintained at 175°C for about three hours. Once all outgassing and evaporation had occurred, the second coating was applied and set at 175°C for about three hours. The resulting thick coating formed on the steel surface is hard,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com