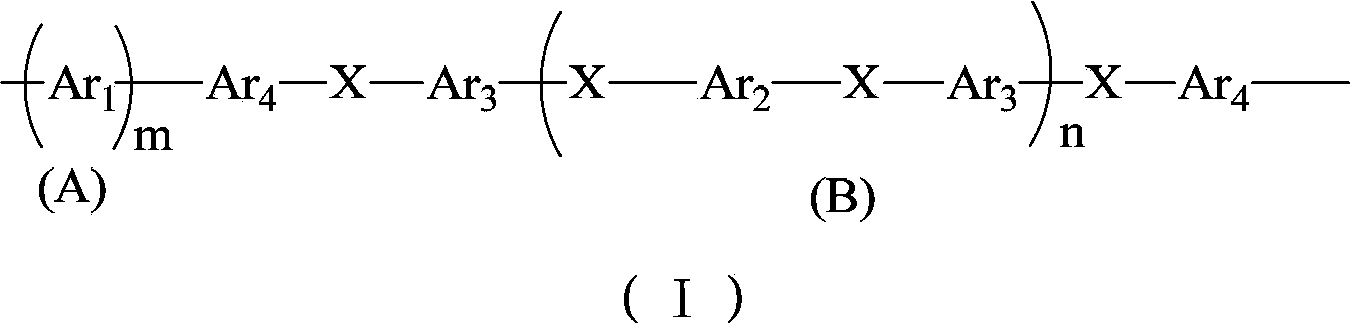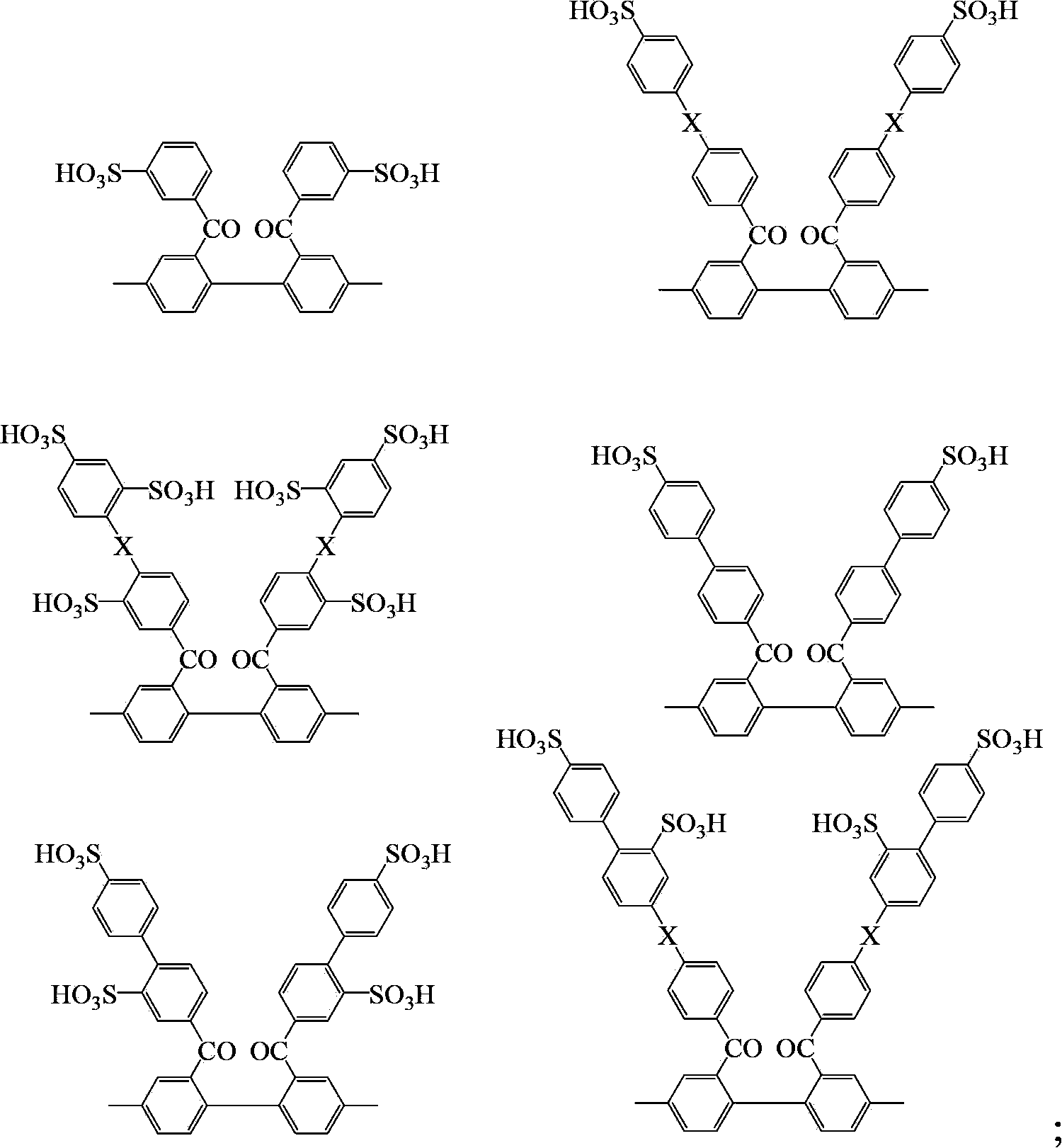Block sulfonated polyphenylene, and preparation method and application thereof
A technology of sulfonated polyimide and sulfonated chain, which is applied in the direction of electrical components, circuits, battery pack parts, etc., can solve the problems of low water resistance, poor mechanical properties, and poor film-forming properties of sulfonated polyimide , to achieve the effect of high proton conductivity, high mechanical strength and excellent chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In this example, the synthesis of sulfonated polyphenylene block polyether ether ketone copolymer is taken as an example. The structural formula is as follows. The specific preparation method includes the following steps:
[0044]
[0045] (1) Preparation of non-sulfonated segment (B) polyether ether ketone with -Cl group as the end group: in N 2 Under protection, first set as Ar 2 monomeric 4,4-dichlorobenzophenone (10 mmol) and as Ar 3 The monomeric 2,2-(4-hydroxybenzene)hexafluoropropane (11 mmol) was dissolved in N,N-dimethylacetamide (DMAC) solvent, and K 2 CO 3 (20mmol), react at 100°C for 2 hours, then react at 160-180°C for 20 hours, and cool to room temperature. At this time, the monomer Ar 2 and Ar 3 After the reaction is completed, an oligomer chain segment terminated by the -OH group is formed, and the length of the oligomer chain segment n=M Ar2 / (M Ar3 -M Ar2 ), wherein M is the number of moles of the monomer, and the length of the oligomer chai...
Embodiment 2
[0049] In this example, the sulfonated polyphenylene block polyetheretherketone copolymer in Example 1 is taken as an example, the structural formula is the same as that in Example 1, and the specific preparation method includes the following steps:
[0050] (1) Preparation of non-sulfonated segment (B) polyether ether ketone with -Cl group as the end group: in N 2 Under protection, first set as Ar 2 monomeric 4,4-difluorobenzophenone (15 mmol) and as Ar 3 Add K 2 CO 3 (20mmol), react at 100°C for 2 hours, then react at 120-140°C for 20 hours, and cool to room temperature. At this time, the monomer Ar 2 and Ar 3 After the reaction is completed, an oligomer chain segment terminated by an -OH group is formed, and the length of the oligomer chain segment is n=15. Adding Ar as a capping group 4 The monomer (4-fluorobenzoyl) p-chlorobenzene was 3.6 mmol, and the temperature of the reaction system was raised to 150°C to continue the reaction for 24 hours.
[0051] The reacti...
Embodiment 3
[0054] This embodiment takes the synthesis of sulfonated polyphenylene block polyoctafluorobiphenyl sulfide copolymer as an example, the structural formula is as follows, and the specific preparation method includes the following steps:
[0055]
[0056] (1) Preparation of non-sulfonated segment (B) polyoctafluorobiphenyl sulfide with -Cl group as the terminal group: in N 2 Under protection, first set as Ar 2 Monomer perfluorobiphenyl (20mmol) and as Ar 3 Monomer 2,2-(4-mercaptophenyl)propane (21mmol) was dissolved in N,N-dimethylformamide solvent, adding K 2 CO 3 (20 mmol), react at 80°C for 2 hours, then react at 120-140°C for 12 hours, and cool to room temperature. At this time, the monomer Ar 2 and Ar 3 After the reaction is completed, an oligomer chain segment terminated by a -SH group is formed, and the length of the chain segment in this embodiment is n=20. Add 2.4 mmol of Ar4 monomer (4-fluorobenzoyl) p-chlorobenzene as a capping group, and raise the temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com