Preparation method of alkali-proof vulcanized fluororubber
A technology of vulcanized fluororubber and fluororubber, which is applied in the field of preparation of organic polymer materials, and can solve difficult problems such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
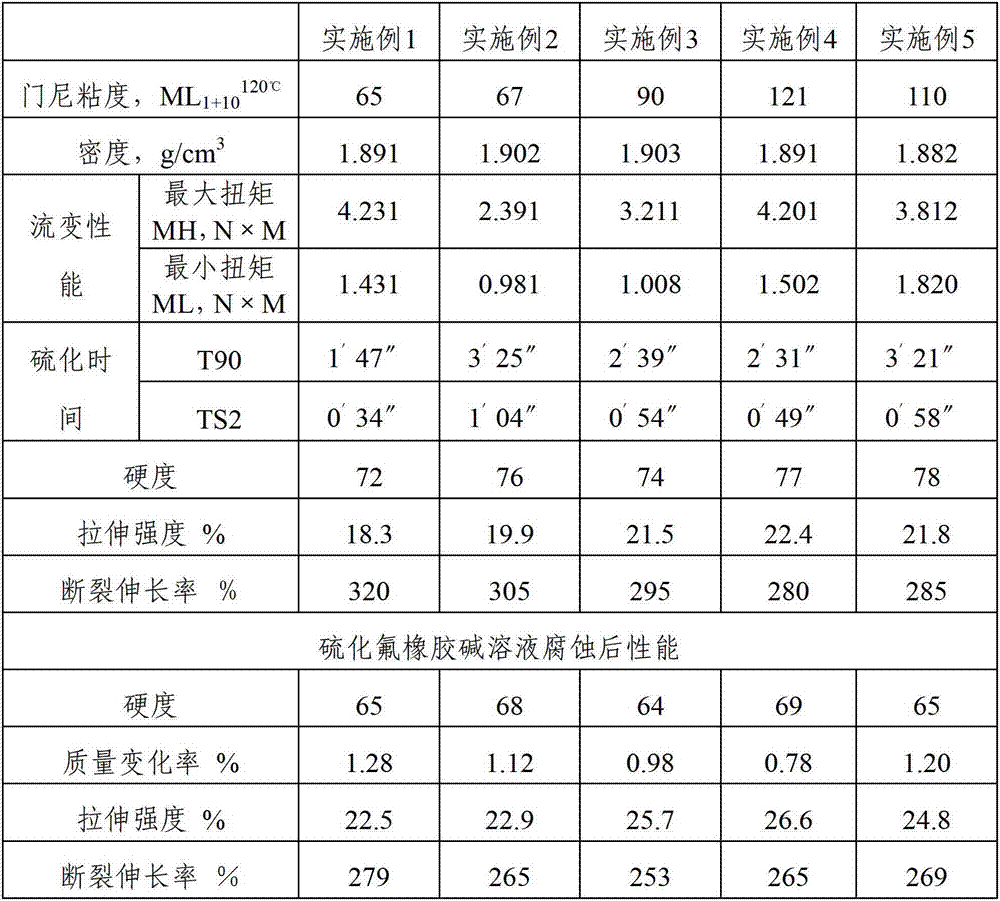
Embodiment 1
[0049] Batch free radical emulsion polymerization was carried out in a 50L autoclave to prepare fluororubber. Add 30L deionized water and 100g 5wt% ammonium perfluorooctanoate aqueous solution in the reactor, 20g pH regulator disodium hydrogen phosphate, the air in the vapor space in the reactor is first replaced with nitrogen, and then replaced with mixed monomers, so that the oxygen content is less than 20ppm, the temperature of the reactor was raised to 120°C. The polymerized monomer mixture was added into the reactor with a diaphragm compressor to raise the pressure to 3.5 MPa. After reaching 3.5MPa, the reactor pressure controller is set to automatically operate at 3.5MPa. The polymerized monomer is composed of 45% vinylidene fluoride, 25% hexafluoropropylene and 22.5% tetrafluoroethylene, 1.5% vulcanization point monomer BVE, 6% monomer CH 2 =CH-CH 3 Composition, the total weight is 12Kg.
[0050] When the pressure of the reactor reaches 3.5MPa, start stirring, fully...
Embodiment 2
[0055]Batch free radical emulsion polymerization was carried out in a 50L autoclave to prepare fluororubber. Add 30L deionized water and 100g 4wt% perfluorooctanoic acid ammonium aqueous solution in reactor, 20g pH adjusting agent borax, the air in steam space in reactor is replaced with nitrogen earlier, then with mixed monomer replacement, make oxygen content less than 25ppm, will The temperature of the reactor was raised to 110°C. Add the polymerization monomer mixture into the reaction kettle with a diaphragm compressor to raise the pressure to 3.5Mpa. After reaching 3.5Mpa, the reactor pressure controller is set at 3.5MPa for automatic operation. The polymerized monomer is composed of 37% vinylidene fluoride, 15% chlorotrifluoroethylene and 22.5% tetrafluoroethylene by mole, and 1.5% vulcanization point monomer CF 2 = CFBr, 3% CH 2 =CH-CH 2 CH 3 , with a total weight of 12Kg.
[0056] When the pressure of the reactor reaches 4.5MPa, start stirring, fully mix the mix...
Embodiment 3
[0060] Batch free radical emulsion polymerization was carried out in a 50L autoclave to prepare fluororubber. Add 30L deionized water and 100g 6wt% ammonium perfluorooctanoate aqueous solution in the reactor, 20g pH adjuster dipotassium hydrogen phosphate, the air in the vapor space in the reactor is first replaced with nitrogen, and then replaced with mixed monomers, so that the oxygen content is less than 25ppm, the temperature of the reactor was raised to 70°C. The polymerized monomer mixture was added into the reactor with a diaphragm compressor to raise the pressure to 2.5 MPa. After reaching 3.5MPa, the reactor pressure controller is set to automatically operate at 3.5MPa. The polymerized monomer is composed of 40% vinylidene fluoride, 25% perfluoropropyl vinyl ether (PPVE) and 23.5% tetrafluoroethylene by mole, and 1.5% vulcanization point monomer CF 2 =CFI, monomer CH 2 =CH-(CH 2 ) 3 -H 10%, the total weight is 12Kg.
[0061] When the pressure of the reactor reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com